Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help plz Given the isotopic symbol for the element Michiganite, provide the requested information: 125251Mi3+ - Blank #1: The number of protons present is: -
help plz 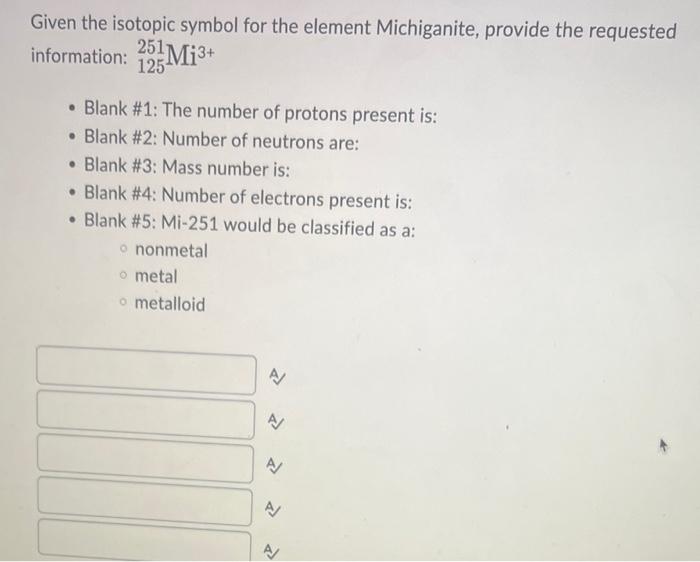
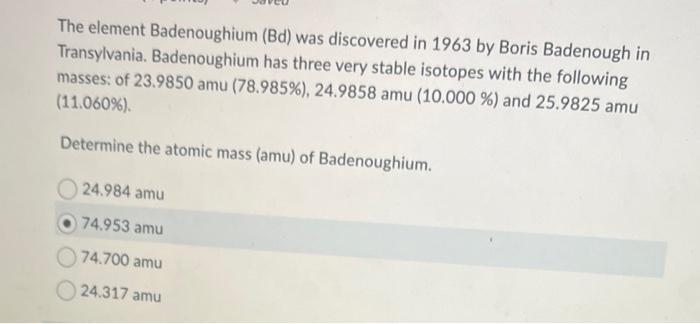
Given the isotopic symbol for the element Michiganite, provide the requested information: 125251Mi3+ - Blank \#1: The number of protons present is: - Blank \#2: Number of neutrons are: - Blank \#3: Mass number is: - Blank \#4: Number of electrons present is: - Blank \#5: Mi-251 would be classified as a: nonmetal metal metalloid The element Badenoughium (Bd) was discovered in 1963 by Boris Badenough in Transylvania. Badenoughium has three very stable isotopes with the following masses: of 23.9850amu(78.985%),24.9858amu(10.000%) and 25.9825amu (11.060\%). Determine the atomic mass (amu) of Badenoughium. 24.984amu 74.953amu 74.700amu 24.317amu 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


