Answered step by step
Verified Expert Solution
Question
1 Approved Answer
HELP!! The vapor pressure, P. of a certain liquid was measured at two temperatures, T. The data is shown in the table. T(K) P (kPa)
HELP!! 
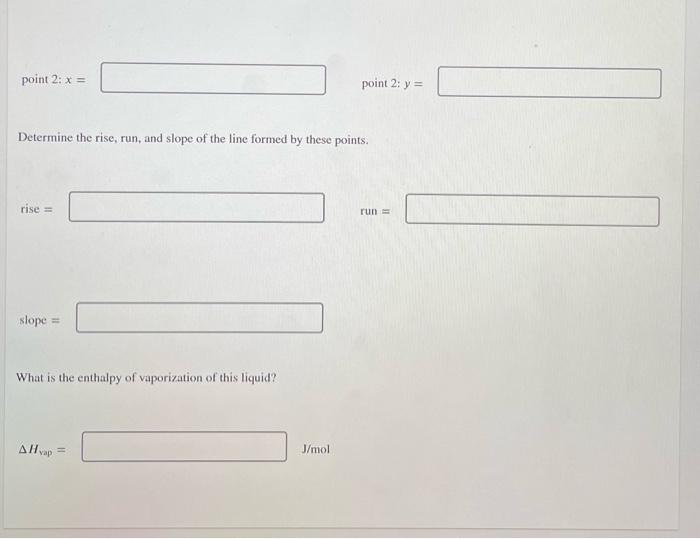
The vapor pressure, P. of a certain liquid was measured at two temperatures, T. The data is shown in the table. T(K) P (kPa) 225 3.44 575 7.08 Keep the pressure units in kilopascals. If you were going to graphically determine the enthalpy of vaporizaton, AH yap, for this liquid, what points would you plot? To avoid rounding errors, use three significant figures in the x-values and four significant figures in the y-values. point 1: x = point 1: y = point 2: x = point 2: y = Determine the rise, run, and slope of the line formed by these points. rise run point 2: x = point 2: y = Determine the rise, run, and slope of the line formed by these points. rise = run slope = What is the enthalpy of vaporization of this liquid? AH vap - J/mol 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


