Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with 5&6 The solubility of O2 in water is 5.7mg/L at an atmospheric pressure of 1.00atm and temperature of 40C. Calculate the Henry's law
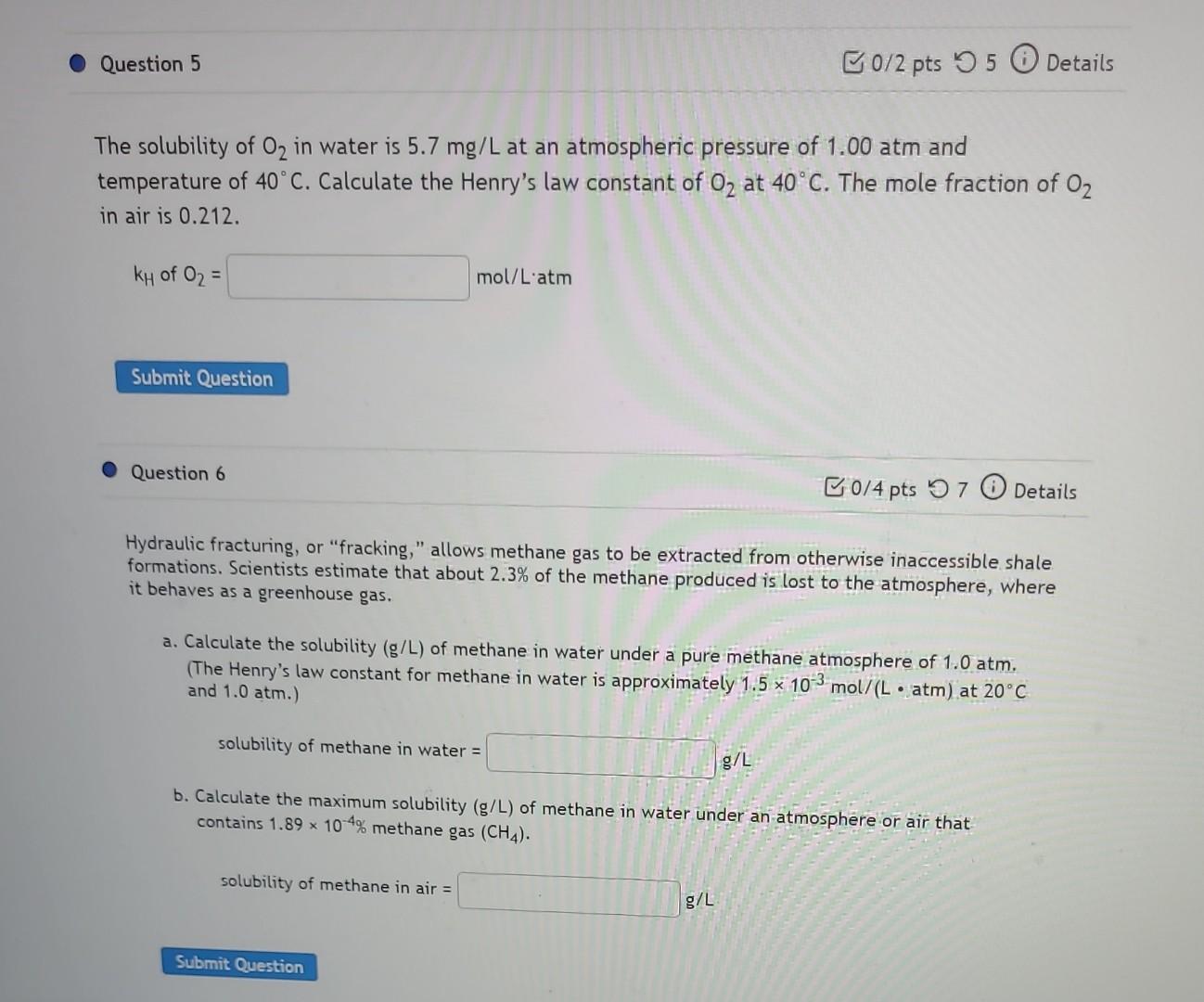
help with 5&6
The solubility of O2 in water is 5.7mg/L at an atmospheric pressure of 1.00atm and temperature of 40C. Calculate the Henry's law constant of O2 at 40C. The mole fraction of O2 in air is 0.212. kHofO2= Question 6 Hydraulic fracturing, or "fracking," allows methane gas to be extracted from otherwise inaccessible shale formations. Scientists estimate that about 2.3% of the methane produced is lost to the atmosphere, where it behaves as a greenhouse gas. a. Calculate the solubility (g/L) of methane in water under a pure methane atmosphere of 1.0atm. (The Henry's law constant for methane in water is approximately 1.5103mol/(Latm) at 20C and 1.0atm.) solubility of methane in water = b. Calculate the maximum solubility (g/L) of methane in water under an atmosphere or air that contains 1.89104% methane gas (CH4). solubility of methane in air =Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


