Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with all please You need to make an aqueous solution of 0.238M ammonium acetate for an experiment in lab, using a 250 mL volumetric
help with all please 
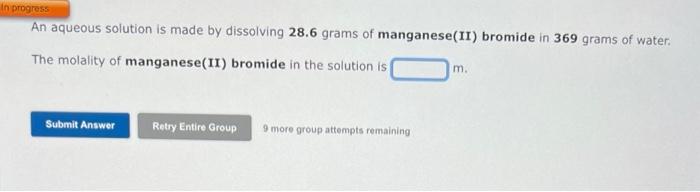
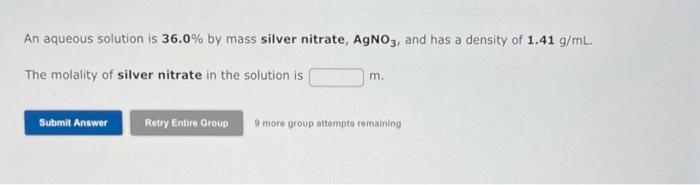
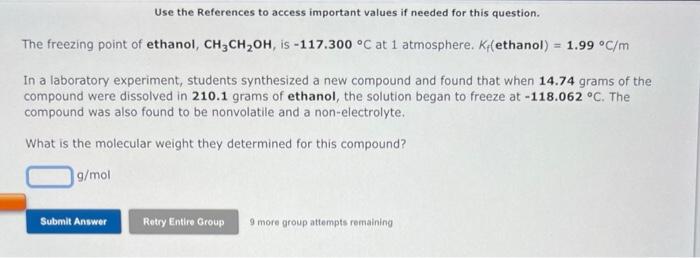 You need to make an aqueous solution of 0.238M ammonium acetate for an experiment in lab, using a 250 mL volumetric flask. How much solid ammonium acetate should you add? grams An aqueous solution is made by dissolving 28.6 grams of manganese(II) bromide in 369 grams of water: The molality of manganese(II) bromide in the solution is 9 more group attempts remaining An aqueous solution is 36.0% by mass silver nitrate, AgNO3, and has a density of 1.41g/mL. The molality of silver nitrate in the solution is m. 9 more group attempts remaining Use the References to access important values if needed for this question. The freezing point of ethanol, CH3CH2OH, is 117.300C at 1 atmosphere. Kf( ethanol) =1.99C/m In a laboratory experiment, students synthesized a new compound and found that when 14.74grams of the compound were dissolved in 210.1 grams of ethanol, the solution began to freeze at 118.062C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol 9 more group attempts remaining
You need to make an aqueous solution of 0.238M ammonium acetate for an experiment in lab, using a 250 mL volumetric flask. How much solid ammonium acetate should you add? grams An aqueous solution is made by dissolving 28.6 grams of manganese(II) bromide in 369 grams of water: The molality of manganese(II) bromide in the solution is 9 more group attempts remaining An aqueous solution is 36.0% by mass silver nitrate, AgNO3, and has a density of 1.41g/mL. The molality of silver nitrate in the solution is m. 9 more group attempts remaining Use the References to access important values if needed for this question. The freezing point of ethanol, CH3CH2OH, is 117.300C at 1 atmosphere. Kf( ethanol) =1.99C/m In a laboratory experiment, students synthesized a new compound and found that when 14.74grams of the compound were dissolved in 210.1 grams of ethanol, the solution began to freeze at 118.062C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol 9 more group attempts remaining




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


