Question
Help with Matlab Code (Membrane reactor) The first-order, gas-phase, reversible reaction is taking place in a membrane reactor. Pure A enters the reactor, and B
Help with Matlab Code
(Membrane reactor) The first-order, gas-phase, reversible reaction is taking place in a membrane reactor. Pure A enters the reactor, and B diffuses out through the membrane. Unfortunately, a small amount of the reactant A also diffuses through the membrane. (a) Plot and analyze the flow rates of A, B, and C and the conversion X down the reactor, as well as the flow rates of A and B through the membrane. (b) Next, compare the conversion profiles in a conventional PFR with those of a membrane reactor from part (a). What generalizations can you make? (c) Would the conversion of A be greater or smaller if C were diffusing out instead of B?
Additional information: k 10 min1 FA0 100 mol/min KC 0.01 mol2/dm6 100 dm3/min kCA 1 min1 Vreactor 20 dm3 kCB 40 min1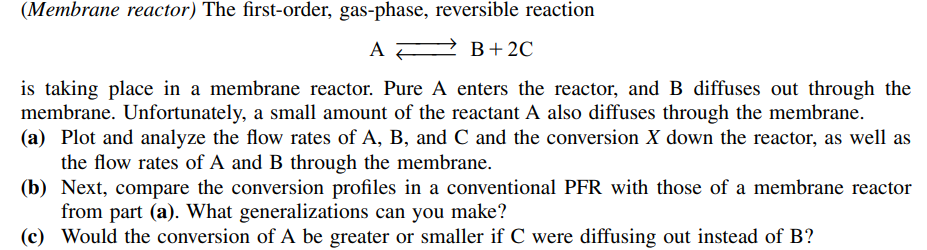
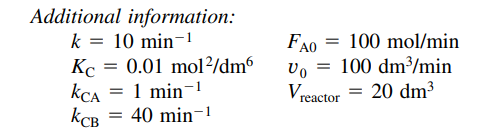
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


