Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help with Part C. Please show all the steps! Thanks 2. Vapor-liquid equilibrium data for mixtures of water and isopropanol at 1 atm are given
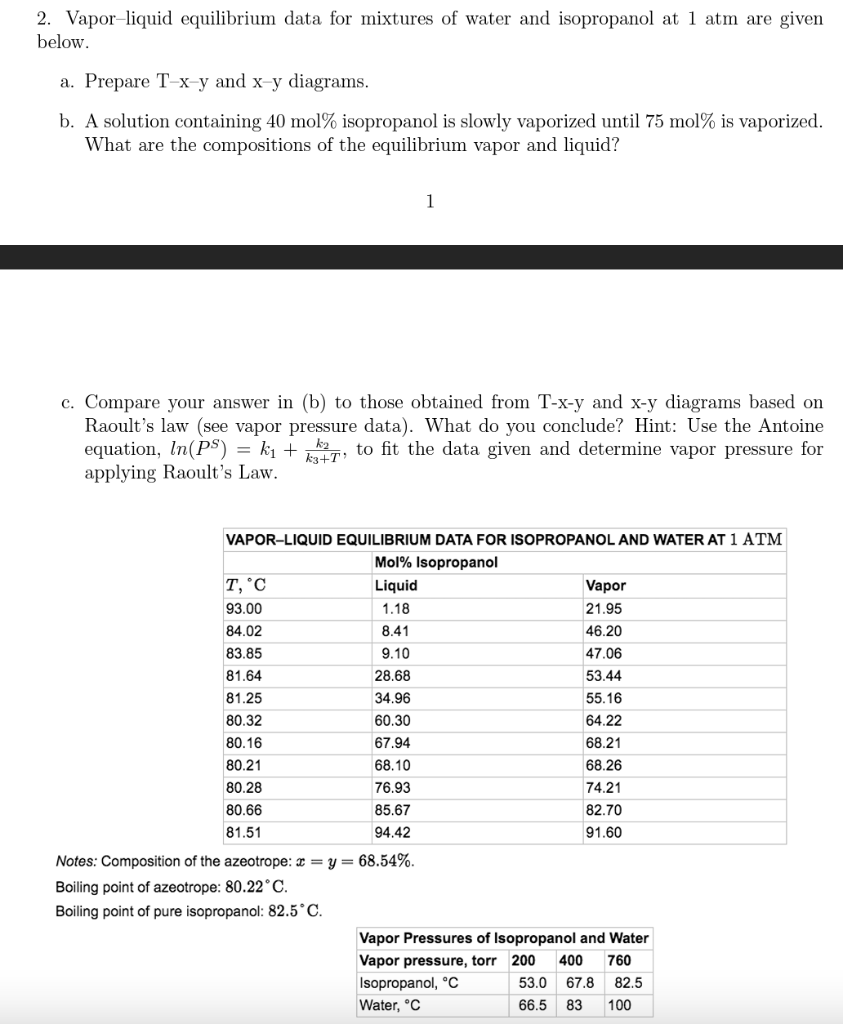
Help with Part C. Please show all the steps! Thanks
2. Vapor-liquid equilibrium data for mixtures of water and isopropanol at 1 atm are given below. a. Prepare Txy and xy diagrams. b. A solution containing 40mol% isopropanol is slowly vaporized until 75mol% is vaporized. What are the compositions of the equilibrium vapor and liquid? 1 c. Compare your answer in (b) to those obtained from T-x-y and x-y diagrams based on Raoult's law (see vapor pressure data). What do you conclude? Hint: Use the Antoine equation, ln(PS)=k1+k3+Tk2, to fit the data given and determine vapor pressure for applying Raoult's Law. Notes: Composition of the azeotrope: x=y=68.54%. Boiling point of azeotrope: 80.22C. Boiling point of pure isopropanol: 82.5CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


