Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with these please Question-3. Given that Pt has an FCC crystal structure, density (P) = 21.45 g/cm', atomic mass (An) = 195.084 g/mol, Avogadro
help with these please 
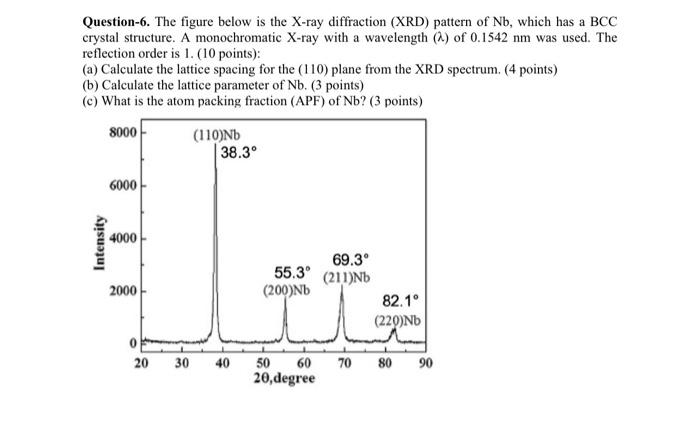
Question-3. Given that Pt has an FCC crystal structure, density (P) = 21.45 g/cm', atomic mass (An) = 195.084 g/mol, Avogadro constant (NA) * 6.022 * 102 atoms/mol. Calculate the radius of the Pt atom. (6 points) Question-6. The figure below is the X-ray diffraction (XRD) pattern of Nb, which has a BCC crystal structure. A monochromatic X-ray with a wavelength (a) of 0.1542 nm was used. The reflection order is 1. (10 points): (a) Calculate the lattice spacing for the (110) plane from the XRD spectrum. (4 points) (b) Calculate the lattice parameter of Nb. (3 points) (c) What is the atom packing fraction (APF) of Nb? (3 points) 8000 (110)ND 38.3 6000 Intensity 4000 69.3 55.39 (211)Nb 2000 (200)ND 82.1 (229)ND 20 30 40 70 80 50 60 20 degree 90 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


