Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with this thanks Introduction: In this experiment you will attempt to experimentally measure Avogadro's number. Avogadro's number is related to the number of molecules
help with this thanks 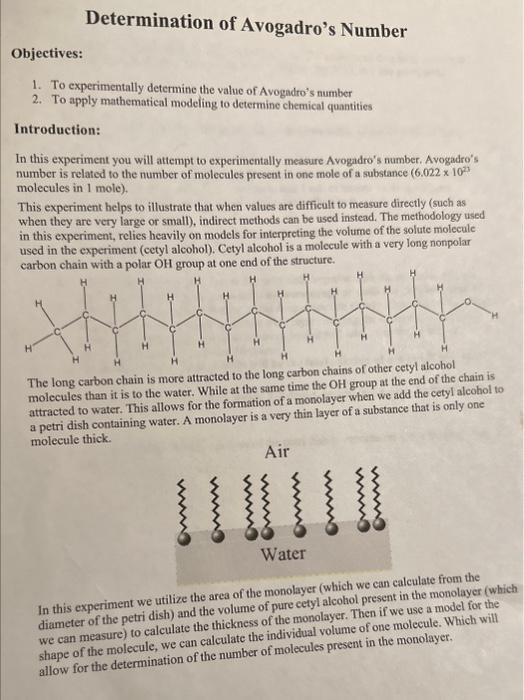




Introduction: In this experiment you will attempt to experimentally measure Avogadro's number. Avogadro's number is related to the number of molecules present in one mole of a substance (6.0221022 molecules in 1 mole). This experiment helps to illustrate that when values are difficult to measure directly (such as when they are very large or small), indirect methods can be used instead. The methodology used in this experiment, relies heavily on models for interpreting the volume of the solute molecule used in the experiment (cetyl alcohol). Cetyl alcohol is a molecule with a very long nonpolar carbon chain with a nolar OH eroun at one end of the structure. The long carbon chain is more attracted to the long earbon cnaurs or unim at the end of the chain is molecules than it is to the water. While at the same time the OH attracted to water. This allows for the formation of a monin layer of a substance that is only one molecule thick. In this experiment we utilize the area of the monolayer (which we can calculate from the diameter of the petri dish) and the volume of pure cetyl alcohol present in the monolayer (which we can measure) to calculate the thickness of the monolayer. Then if we use a model for the shape of the molecule, we can calculate the individual volume of one molecule. Which will allow for the determination of the number of molecules present in the monolayer. 1. Obtain a petri dish from the cart in the lab room. Clean the petri dish with deionized water and then wash with 1mL of 0.1MNH4OH. Do NOT dry the petri dish! 2. Fill the petri dish half full of deionized water. 3. Clean a medicine dropper by rinsing with deionized water, and with 1mL of hexane. 4. Calibrate the medicine dropper: take 6-7 mL of hexane, in a clean 25mL Erlenmeyer flask. Fill a 10mL graduated cylinder with 3mL of hexane, then using the medicine dropper count how many drops it takes to add 1mL of hexane to the graduated cylinder. Repeat this one more time and average the result. 5. Clean your 25mL Erlenmeyer flask and obtain about 1mL of 1.08104g/mL of cetyl alcohol in hexane solution. 6. Add the cetyl alcohol solution to the petri dish dropwise with the medicine dropper that was calibrated. Wait 30 seconds between drops or until the previous drop is gone. You should see the hexane in the drop evaporate slowly. Once a monolayer has formed (where there is one molecule wide layer covering the surface of water in the petri dish), an additional drop will leave a lens or will take more than 1min to disappear (should be less than 10 drops). (Number of drops in data table will be the total number of drops added 1 ). 7. If the monolayer is reached within 2-3 drops, reclean your petri dish and start over. 8. Measure and record the diameter of the petri dish in centimeters (record in the data table). Density of Pure Cetyl Alcohol (D): Volume of Pure Cetyl Alcohol (V=m/D) : Area of Monolayer (A=d2/4) : Thickness of Monolayer (t=V/A) : Calculating Avogadro's Number (Model 1 Cubic Molecules): In this model we will assume that the shape of each molecule of cetyl alcohol is in the shape of a cube. This will allow for the calculation of the volume of the molecule, as we can assume that each side length of the cube is equal to the thickness. Volume of a Molecule of Cetyl Alcohol (v=t3) : Number of Molecules in the Monolayer (M=V/v) : Number of Moles in the Monolayer (n=m/242.50g/mol) : Avogadro's Number (number of molecules in 1mole, No =M ): Calculating Avogadro's Number (Model 2 Rectangular Prism Molecules): In this model we will assume that the shape of each molecule of cetyl alcohol is in the shape of a rectangular prism as seen below. This will again allow for the calculation of the volume of the molecule from its thickness, assuming that height is equal to thickness, and both length and width are equal to one fifth the thickness. This model should be more accurate based on it being closer to the actual shape of cetyl alcohol. Volume of a Molecule of Cetyl Alcohol [v=t(t/5)(t/5)] : Number of Molecules in the Monolayer (M=V/v) : Number of Moles in the Monolayer (n=m/242.50g/mol) : Avogadro's Number (number of molecules in 1mole,No=M ): 1. Comment on the results of the experiment, which model for the volume of a cetyl alcohol molecule was more effective? What could be done to improve the accuracy of the experiment further? 2. A student running the same experiment above decides they want to use a cylindrical model for the molecules. In order for the monolayer to form the student had to add 25 drops of 1.08104g/mL (cetyl alcohol in hexane solution). The diameter of the petri dish used was 17.5cm. The medicine dropper calibration was 81 drops per 1mL. The volume of the cylindrical molecule was assumed to be v=r2h, where the radius was assumed to equal r=t/10, and the height = thickness of the monolayer. What would be the value of Avogadro's number using this model? Introduction: In this experiment you will attempt to experimentally measure Avogadro's number. Avogadro's number is related to the number of molecules present in one mole of a substance (6.0221022 molecules in 1 mole). This experiment helps to illustrate that when values are difficult to measure directly (such as when they are very large or small), indirect methods can be used instead. The methodology used in this experiment, relies heavily on models for interpreting the volume of the solute molecule used in the experiment (cetyl alcohol). Cetyl alcohol is a molecule with a very long nonpolar carbon chain with a nolar OH eroun at one end of the structure. The long carbon chain is more attracted to the long earbon cnaurs or unim at the end of the chain is molecules than it is to the water. While at the same time the OH attracted to water. This allows for the formation of a monin layer of a substance that is only one molecule thick. In this experiment we utilize the area of the monolayer (which we can calculate from the diameter of the petri dish) and the volume of pure cetyl alcohol present in the monolayer (which we can measure) to calculate the thickness of the monolayer. Then if we use a model for the shape of the molecule, we can calculate the individual volume of one molecule. Which will allow for the determination of the number of molecules present in the monolayer. 1. Obtain a petri dish from the cart in the lab room. Clean the petri dish with deionized water and then wash with 1mL of 0.1MNH4OH. Do NOT dry the petri dish! 2. Fill the petri dish half full of deionized water. 3. Clean a medicine dropper by rinsing with deionized water, and with 1mL of hexane. 4. Calibrate the medicine dropper: take 6-7 mL of hexane, in a clean 25mL Erlenmeyer flask. Fill a 10mL graduated cylinder with 3mL of hexane, then using the medicine dropper count how many drops it takes to add 1mL of hexane to the graduated cylinder. Repeat this one more time and average the result. 5. Clean your 25mL Erlenmeyer flask and obtain about 1mL of 1.08104g/mL of cetyl alcohol in hexane solution. 6. Add the cetyl alcohol solution to the petri dish dropwise with the medicine dropper that was calibrated. Wait 30 seconds between drops or until the previous drop is gone. You should see the hexane in the drop evaporate slowly. Once a monolayer has formed (where there is one molecule wide layer covering the surface of water in the petri dish), an additional drop will leave a lens or will take more than 1min to disappear (should be less than 10 drops). (Number of drops in data table will be the total number of drops added 1 ). 7. If the monolayer is reached within 2-3 drops, reclean your petri dish and start over. 8. Measure and record the diameter of the petri dish in centimeters (record in the data table). Density of Pure Cetyl Alcohol (D): Volume of Pure Cetyl Alcohol (V=m/D) : Area of Monolayer (A=d2/4) : Thickness of Monolayer (t=V/A) : Calculating Avogadro's Number (Model 1 Cubic Molecules): In this model we will assume that the shape of each molecule of cetyl alcohol is in the shape of a cube. This will allow for the calculation of the volume of the molecule, as we can assume that each side length of the cube is equal to the thickness. Volume of a Molecule of Cetyl Alcohol (v=t3) : Number of Molecules in the Monolayer (M=V/v) : Number of Moles in the Monolayer (n=m/242.50g/mol) : Avogadro's Number (number of molecules in 1mole, No =M ): Calculating Avogadro's Number (Model 2 Rectangular Prism Molecules): In this model we will assume that the shape of each molecule of cetyl alcohol is in the shape of a rectangular prism as seen below. This will again allow for the calculation of the volume of the molecule from its thickness, assuming that height is equal to thickness, and both length and width are equal to one fifth the thickness. This model should be more accurate based on it being closer to the actual shape of cetyl alcohol. Volume of a Molecule of Cetyl Alcohol [v=t(t/5)(t/5)] : Number of Molecules in the Monolayer (M=V/v) : Number of Moles in the Monolayer (n=m/242.50g/mol) : Avogadro's Number (number of molecules in 1mole,No=M ): 1. Comment on the results of the experiment, which model for the volume of a cetyl alcohol molecule was more effective? What could be done to improve the accuracy of the experiment further? 2. A student running the same experiment above decides they want to use a cylindrical model for the molecules. In order for the monolayer to form the student had to add 25 drops of 1.08104g/mL (cetyl alcohol in hexane solution). The diameter of the petri dish used was 17.5cm. The medicine dropper calibration was 81 drops per 1mL. The volume of the cylindrical molecule was assumed to be v=r2h, where the radius was assumed to equal r=t/10, and the height = thickness of the monolayer. What would be the value of Avogadro's number using this model 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


