Answered step by step
Verified Expert Solution
Question
1 Approved Answer
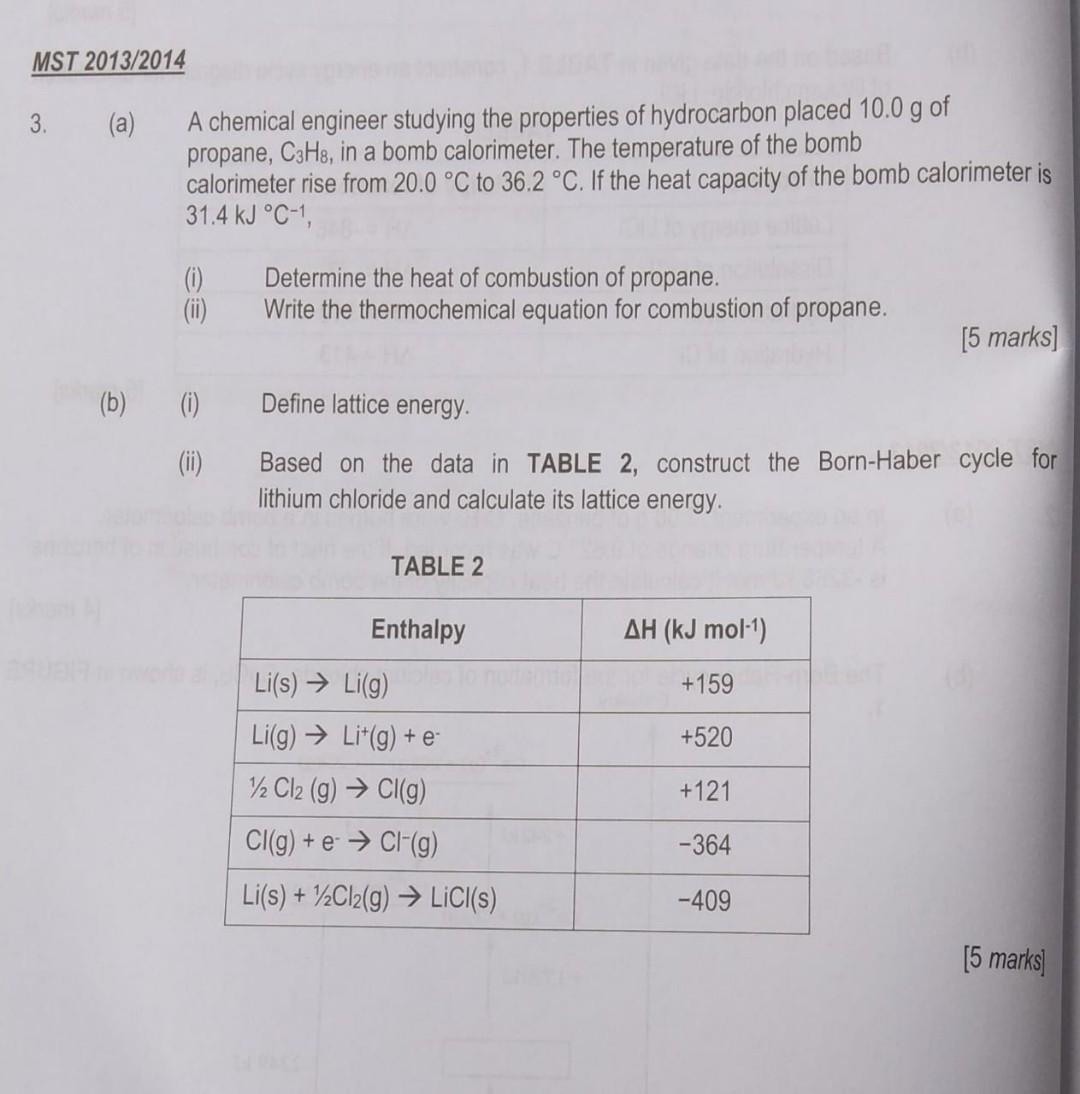
helppp (a) A chemical engineer studying the properties of hydrocarbon placed 10.0g of propane, C3H8, in a bomb calorimeter. The temperature of the bomb calorimeter
helppp
(a) A chemical engineer studying the properties of hydrocarbon placed 10.0g of propane, C3H8, in a bomb calorimeter. The temperature of the bomb calorimeter rise from 20.0C to 36.2C. If the heat capacity of the bomb calorimeter is 31.4kJCC1, (i) Determine the heat of combustion of propane. (ii) Write the thermochemical equation for combustion of propane. [5 marks] (b) (i) Define lattice energy. (ii) Based on the data in TABLE 2, construct the Born-Haber cycle for lithium chloride and calculate its lattice energy. TABLE 2 [5 marks]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


