Answered step by step
Verified Expert Solution
Question
1 Approved Answer
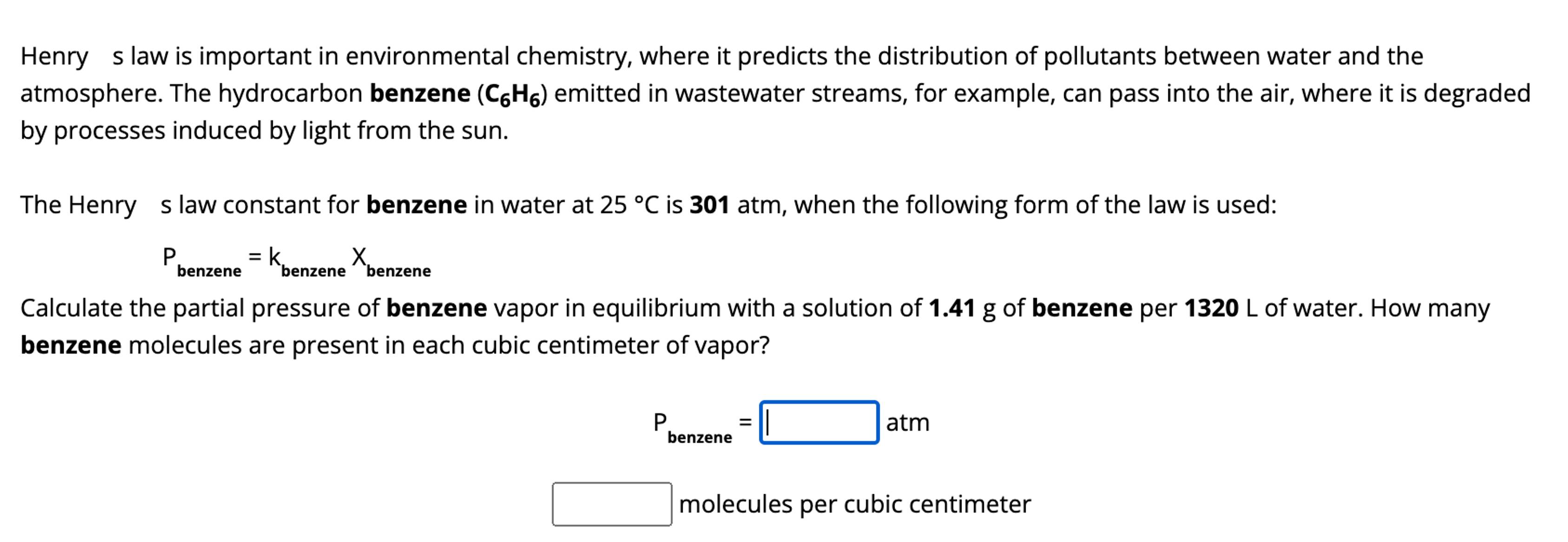
Henry s law is important in environmental chemistry, where it predicts the distribution of pollutants between water and the atmosphere. The hydrocarbon benzene ( C
Henry s law is important in environmental chemistry, where it predicts the distribution of pollutants between water and the
atmosphere. The hydrocarbon benzene emitted in wastewater streams, for example, can pass into the air, where it is degraded
by processes induced by light from the sun.
The Henry s law constant for benzene in water at is atm, when the following form of the law is used:
Calculate the partial pressure of benzene vapor in equilibrium with a solution of of benzene per of water. How many
benzene molecules are present in each cubic centimeter of vapor?
atm
molecules per cubic centimeter
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


