Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Here is my problem:In the absence of turbulent mixing, the partial pressure of each constituent of air would fall off with height above sea level
Here is my problem:In the absence of turbulent mixing, the partial pressure of each
constituent of air would fall off with height above sea level in
Earth's atmosphere as where is the partial
pressure at the height is the partial pressure of component at
sea level, is the acceleration of gravity, is the gas constant, is
the absolute temperature, and is the molecular mass of the gas.
As a result of turbulent mixing, the composition of Earth's
atmosphere is constant below an altitude of but the total
pressure decreases with altitude as where
is the mean molecular weight of air. At sea level,
and and Calculate the total pressure at assuming a mean molecular mass of and that throughout this altitude range.
Express the total pressure in pascals to three significant figures.
Incorrect; Try Again; attempts remaining
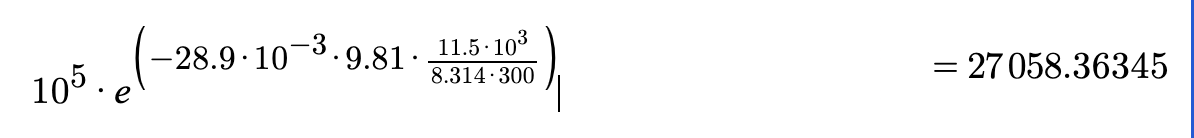
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


