Answered step by step
Verified Expert Solution
Question
1 Approved Answer
here is the translation : 4. Consider the oxidation of solid magnesium. Calculate DeltaG, DeltaH and DeltaS at 298k. Use the following values (excerpted from
here is the translation : 4. Consider the oxidation of solid magnesium. Calculate DeltaG, DeltaH and DeltaS at 298k. Use the following values (excerpted from the Table E by Kubaschewski and Evans): 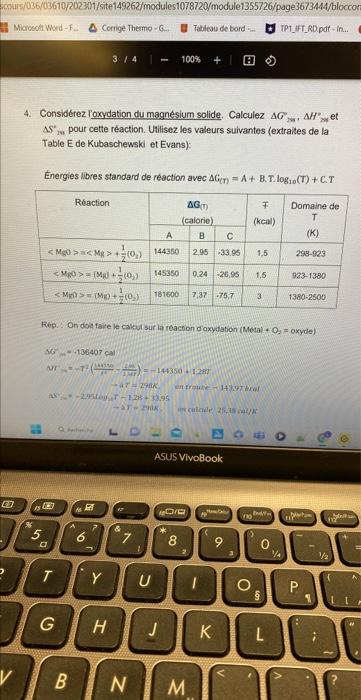 the answers are at the end 4. Considrez loxydation du magnsium solide. Calculez G,H ze et AS in pour cette raction. Uilisez les valeurs suivantes (extraites de la Table E de Kubaschewski et Evans): Energies libres standard de raction avec G(T)=A+B.T.log10(T)+C.T Rep. On doa taire le cakui sur la reacton doxydation (Motal + O2= oxyde)
the answers are at the end 4. Considrez loxydation du magnsium solide. Calculez G,H ze et AS in pour cette raction. Uilisez les valeurs suivantes (extraites de la Table E de Kubaschewski et Evans): Energies libres standard de raction avec G(T)=A+B.T.log10(T)+C.T Rep. On doa taire le cakui sur la reacton doxydation (Motal + O2= oxyde)
here is the translation :
4. Consider the oxidation of solid magnesium. Calculate DeltaG, DeltaH and DeltaS at 298k.
Use the following values (excerpted from the
Table E by Kubaschewski and Evans):

the answers are at the end
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


