Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Here, we imagine a reactor designed along the lines suggested in the following figure, which has a rectangular cross section. Gases are conveyed to the
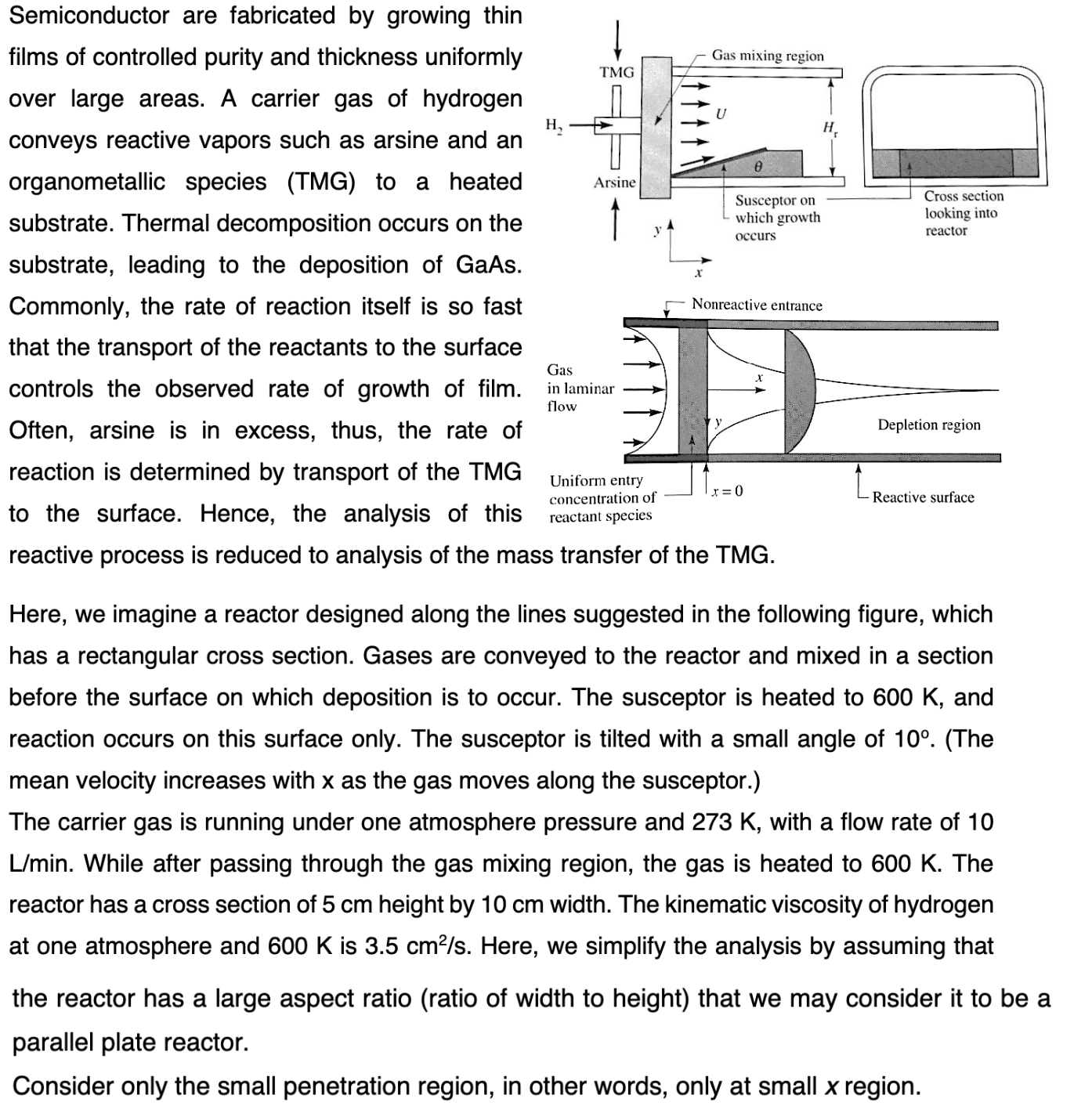
 Here, we imagine a reactor designed along the lines suggested in the following figure, which has a rectangular cross section. Gases are conveyed to the reactor and mixed in a section before the surface on which deposition is to occur. The susceptor is heated to 600K, and reaction occurs on this surface only. The susceptor is tilted with a small angle of 10. (The mean velocity increases with x as the gas moves along the susceptor.) The carrier gas is running under one atmosphere pressure and 273K, with a flow rate of 10 L/min. While after passing through the gas mixing region, the gas is heated to 600K. The reactor has a cross section of 5cm height by 10cm width. The kinematic viscosity of hydrogen at one atmosphere and 600K is 3.5cm2/s. Here, we simplify the analysis by assuming that the reactor has a large aspect ratio (ratio of width to height) that we may consider it to be parallel plate reactor. Consider only the small penetration region, in other words, only at small x region. a) Solve for the concentration profile. b) Write the local flux of reactant at the reactive surface
Here, we imagine a reactor designed along the lines suggested in the following figure, which has a rectangular cross section. Gases are conveyed to the reactor and mixed in a section before the surface on which deposition is to occur. The susceptor is heated to 600K, and reaction occurs on this surface only. The susceptor is tilted with a small angle of 10. (The mean velocity increases with x as the gas moves along the susceptor.) The carrier gas is running under one atmosphere pressure and 273K, with a flow rate of 10 L/min. While after passing through the gas mixing region, the gas is heated to 600K. The reactor has a cross section of 5cm height by 10cm width. The kinematic viscosity of hydrogen at one atmosphere and 600K is 3.5cm2/s. Here, we simplify the analysis by assuming that the reactor has a large aspect ratio (ratio of width to height) that we may consider it to be parallel plate reactor. Consider only the small penetration region, in other words, only at small x region. a) Solve for the concentration profile. b) Write the local flux of reactant at the reactive surface Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


