Question
Here we will evaluate an elementary, exothermic, irreversible reaction in a tubular reactor (liquid phase, laminar flow regime). The reactor uses a constant temperature cooling

 Here we will evaluate an elementary, exothermic, irreversible reaction in a tubular reactor (liquid phase, laminar flow regime). The reactor uses a constant temperature cooling jacket to keep its temperature down. The steady-state behavior of the reactor will be investigated. The reaction kinetics and physical properties of the species are provided below. The Model Definition section provides a general description of the complete reactor model, whereas the Modeling Instructions details how to set up and solve the model.
Here we will evaluate an elementary, exothermic, irreversible reaction in a tubular reactor (liquid phase, laminar flow regime). The reactor uses a constant temperature cooling jacket to keep its temperature down. The steady-state behavior of the reactor will be investigated. The reaction kinetics and physical properties of the species are provided below. The Model Definition section provides a general description of the complete reactor model, whereas the Modeling Instructions details how to set up and solve the model.
Model Definition
Reaction
The reaction is a IRREVERSIBLE conversion of species A, B, and C in liquid.
A + B C (1)
A is the notation for propylene oxide, B water, and C propylene glycol. B is in excess and is modeled as a solvent. The reaction kinetics is 1st order in regard to the concentration of A:
r_1 = k_1*c_A
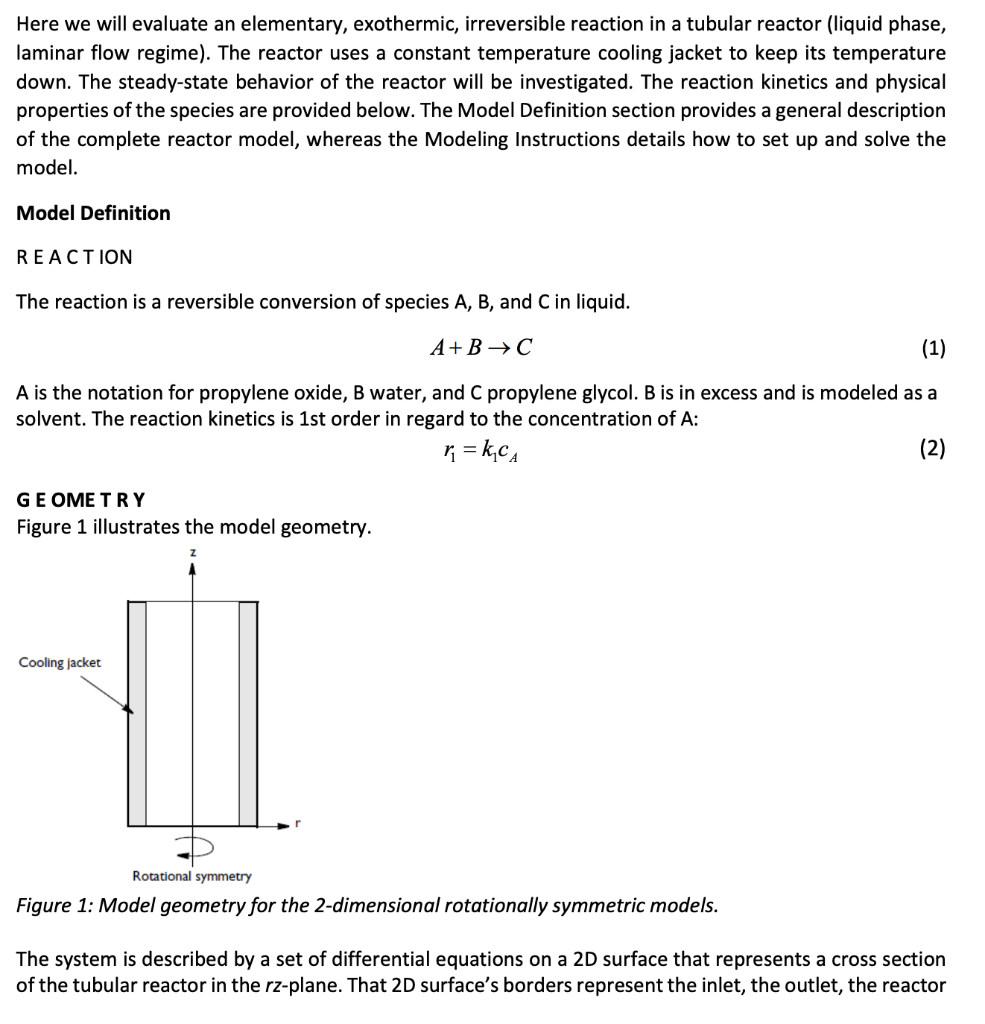
The system is described by a set of differential equations on a 2D surface that represents a cross section of the tubular reactor in the rz-plane. That 2D surfaces borders represent the inlet, the outlet, the reactor wall, and the centerline. The reactor model uses the following differential equations; mass balances for the species. Due to rotational symmetry, you need only to solve these equations for half of the domain shown in Figure 1.
MODEL EQUATIONS
You can describe the mass balances in the reactors with partial differential equations (PDEs). The equations are defined as follows.
where Dpi denotes the diffusion coefficient, Ci the concentration, U the flow velocity, Ra the radius of the reactor, and Ri is the reaction rate.
Assume parabolic laminar flow profile.
Complete the Four Tasks below
1. Implement FDM and create a MATLAB function file to obtain coefficient matrix A and non-linear vector b for the ordinary differential equation below dc/dz = Ac + b
2. Write a MATLAB file to solve the ordinary differential equation above.
3. Plot the concentration profiles c_A(z,r). Choose 200 equally spaced points in the r-direction.
Here we will evaluate an elementary, exothermic, irreversible reaction in a tubular reactor (liquid phase, laminar flow regime). The reactor uses a constant temperature cooling jacket to keep its temperature down. The steady-state behavior of the reactor will be investigated. The reaction kinetics and physical properties of the species are provided below. The Model Definition section provides a general description of the complete reactor model, whereas the Modeling Instructions details how to set up and solve the model. Model Definition REACTION The reaction is a reversible conversion of species A, B, and C in liquid. A+B C (1) A is the notation for propylene oxide, B water, and C propylene glycol. B is in excess and is modeled as a solvent. The reaction kinetics is 1st order in regard to the concentration of A: n = kca (2) GEOMETRY Figure 1 illustrates the model geometry. Cooling jacket Rotational symmetry Figure 1: Model geometry for the 2-dimensional rotationally symmetric models. The system is described by a set of differential equations on a 2D surface that represents a cross section of the tubular reactor in the rz-plane. That 2D surface's borders represent the inlet, the outlet, the reactor wall, and the centerline. The reactor model uses the following differential equations; mass balances for the species. Due to rotational symmetry, you need only to solve these equations for half of the domain shown in Figure 1. MODEL EQUATIONS You can describe the mass balances in the reactors with partial differential equations (PDEs). The equations are defined as follows. Mass Balance 10C; D +D Prar -2U14 ( r R +R= 0 (3) P where Dpi denotes the diffusion coefficient, C; the concentration, U the flow velocity, Ra the radius of the reactor, and R, is the reaction rate. Mass Balance Boundary Conditions Inlet (z = 0) . C;(r,0) = Cio At the wall (r =R) OCA (R, 2) = 0 ar Outlet (z = L) OCA(r, L) = 0 where L denotes the length of the reactor. Assume parabolic laminar flow profile. The constants in the model are: s . Activation energy, E = 75362 J/mol Frequency factor, A = 16.96E12 1/h Thermal conductivity of B, ke = 0.559 W/(m-K) Overall heat-transfer coefficient, Uk = 1300 W/(mK) Inlet temperature, TO = 312 K Temperature of the coolant, Tal = 273 K Heat of reaction, AH Rx, dHrx = -84666 J/mol Average fluid flow rate, u0 = 0.002 m3/s Concentration A at inlet, cao = rho_po_p/M_po* (1/9) mol/m3 Concentration B, CBO = rho_w_p/M_w* (7/9) mol/m3 Molar heat capacity B, cpm_B = 74.5 J/(molK) Reactor radius, Ra = 0.1 m Reactor length, L = 1 m Density, A, rho_A = 830 kg/m3 Density, B, rho_B = 1000 kg/m3 Density, C, rho_C = 1040 kg/m3 Reference dynamic viscosity of B (at 293 K), myref_B = 1 mPa.s The conversion of species A is given by Complete the Four Tasks below- . . 1. 20 points - Implement FDM and create a MATLAB function file to obtain coefficient matrix A and non-linear vector b for the ordinary differential equation below dc = Ac+b dz 2. 20 points - Write a MATLAB file to solve the ordinary differential equation above. 3. 40 points Plot the concentration profiles ca (z,r). Choose 200 equally spaced points in the r-direction. Here we will evaluate an elementary, exothermic, irreversible reaction in a tubular reactor (liquid phase, laminar flow regime). The reactor uses a constant temperature cooling jacket to keep its temperature down. The steady-state behavior of the reactor will be investigated. The reaction kinetics and physical properties of the species are provided below. The Model Definition section provides a general description of the complete reactor model, whereas the Modeling Instructions details how to set up and solve the model. Model Definition REACTION The reaction is a reversible conversion of species A, B, and C in liquid. A+B C (1) A is the notation for propylene oxide, B water, and C propylene glycol. B is in excess and is modeled as a solvent. The reaction kinetics is 1st order in regard to the concentration of A: n = kca (2) GEOMETRY Figure 1 illustrates the model geometry. Cooling jacket Rotational symmetry Figure 1: Model geometry for the 2-dimensional rotationally symmetric models. The system is described by a set of differential equations on a 2D surface that represents a cross section of the tubular reactor in the rz-plane. That 2D surface's borders represent the inlet, the outlet, the reactor wall, and the centerline. The reactor model uses the following differential equations; mass balances for the species. Due to rotational symmetry, you need only to solve these equations for half of the domain shown in Figure 1. MODEL EQUATIONS You can describe the mass balances in the reactors with partial differential equations (PDEs). The equations are defined as follows. Mass Balance 10C; D +D Prar -2U14 ( r R +R= 0 (3) P where Dpi denotes the diffusion coefficient, C; the concentration, U the flow velocity, Ra the radius of the reactor, and R, is the reaction rate. Mass Balance Boundary Conditions Inlet (z = 0) . C;(r,0) = Cio At the wall (r =R) OCA (R, 2) = 0 ar Outlet (z = L) OCA(r, L) = 0 where L denotes the length of the reactor. Assume parabolic laminar flow profile. The constants in the model are: s . Activation energy, E = 75362 J/mol Frequency factor, A = 16.96E12 1/h Thermal conductivity of B, ke = 0.559 W/(m-K) Overall heat-transfer coefficient, Uk = 1300 W/(mK) Inlet temperature, TO = 312 K Temperature of the coolant, Tal = 273 K Heat of reaction, AH Rx, dHrx = -84666 J/mol Average fluid flow rate, u0 = 0.002 m3/s Concentration A at inlet, cao = rho_po_p/M_po* (1/9) mol/m3 Concentration B, CBO = rho_w_p/M_w* (7/9) mol/m3 Molar heat capacity B, cpm_B = 74.5 J/(molK) Reactor radius, Ra = 0.1 m Reactor length, L = 1 m Density, A, rho_A = 830 kg/m3 Density, B, rho_B = 1000 kg/m3 Density, C, rho_C = 1040 kg/m3 Reference dynamic viscosity of B (at 293 K), myref_B = 1 mPa.s The conversion of species A is given by Complete the Four Tasks below- . . 1. 20 points - Implement FDM and create a MATLAB function file to obtain coefficient matrix A and non-linear vector b for the ordinary differential equation below dc = Ac+b dz 2. 20 points - Write a MATLAB file to solve the ordinary differential equation above. 3. 40 points Plot the concentration profiles ca (z,r). Choose 200 equally spaced points in the r-directionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


