Answered step by step
Verified Expert Solution
Question
1 Approved Answer
heres the full thing can you do 3. the reaction The data provided in tables 1 and 2 below were collected during the culminating lab.

heres the full thing can you do 3. 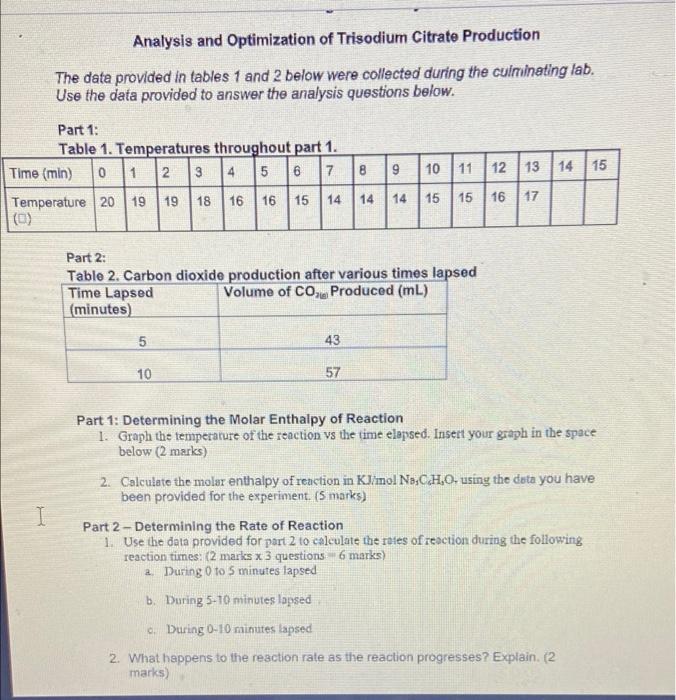

the reaction 
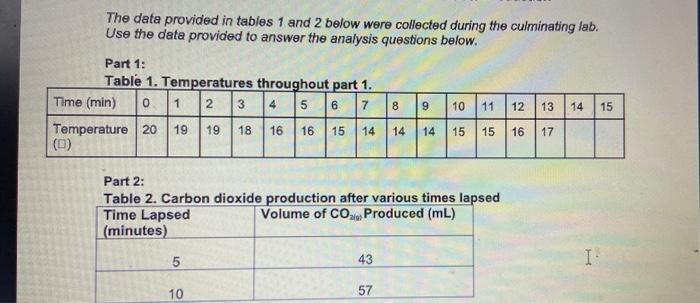
The data provided in tables 1 and 2 below were collected during the culminating lab. Use the data provided to answer the analysis questions below. Part 1: Table 1. Temperatures throughout part 1. Time (min) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Temperature 20 19 19 18 16 16 15 14 14 14 15 15 16 17 (0) 14 15 Part 2: Table 2. Carbon dioxide production after various times lapsed Time Lapsed Volume of CO2le Produced (mL) (minutes) 5 43 I 10 57 3. Draw a potential energy diagram for the reaction. Label the following on your diagram: axes with units, enthalpy of products, enthalpy of reactants, enthalpy change, activated complex, activation energy of the forward reaction, activation energy of the reverse reaction. (3 marks) Analysis and Optimization of Trisodium Citrate Production The data provided in tables 1 and 2 below were collected during the culminating lab. Use the data provided to answer the analysis questions below. Part 1: Table 1. Temperatures throughout part 1. Time (min) 0 1 2 314 5 6 7 8 9 10 | 11 12 13 14 15 19 18 16 16 15 Temperature 20 19 14 14 16 16 14 15 15 16 17 Part 2: Table 2. Carbon dioxide production after various times lapsed Time Lapsed Volume of CO Produced (mL) (minutes) 5 43 10 57 I Part 1: Determining the Molar Enthalpy of Reaction 1. Graph the temperature of the reaction vs the time elapsed. Insert your graph in the space below (2 marks) 2. Calculate the molar enthalpy of reaction in KJ/mol NCH,0- using the data you have been provided for the experiment. (5 marks) Part 2 - Determining the Rate of Reaction 1. Use the data provided for part 2 to calculate the roles of reaction during the following reaction times: (2 marks x 3 questions 6 marks) 2. During 0 to 5 minutes lapsed b. During 5-10 minutes lapsed During 0-10 minutes lapsed 2. What happens to the reaction rate as the reaction progresses? Explain. (2 marks) Introduction: In the presence of water, citric acid (C,H,O,) and sodium bicarbonate (NaHCO3) (also known as baking soda) react to form trisodium citrate (Na,C,H,O;), water (H,0) and carbon dioxide (CO). C.M.Q. +3NaHCO, No,C,H,O, +31,01300, Vahiralarenereerible doen in The data provided in tables 1 and 2 below were collected during the culminating lab. Use the data provided to answer the analysis questions below. Part 1: Table 1. Temperatures throughout part 1. Time (min) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Temperature 20 19 19 18 16 16 15 14 14 14 15 15 16 17 (0) 14 15 Part 2: Table 2. Carbon dioxide production after various times lapsed Time Lapsed Volume of CO2le Produced (mL) (minutes) 5 43 I 10 57 3. Draw a potential energy diagram for the reaction. Label the following on your diagram: axes with units, enthalpy of products, enthalpy of reactants, enthalpy change, activated complex, activation energy of the forward reaction, activation energy of the reverse reaction. (3 marks) Analysis and Optimization of Trisodium Citrate Production The data provided in tables 1 and 2 below were collected during the culminating lab. Use the data provided to answer the analysis questions below. Part 1: Table 1. Temperatures throughout part 1. Time (min) 0 1 2 314 5 6 7 8 9 10 | 11 12 13 14 15 19 18 16 16 15 Temperature 20 19 14 14 16 16 14 15 15 16 17 Part 2: Table 2. Carbon dioxide production after various times lapsed Time Lapsed Volume of CO Produced (mL) (minutes) 5 43 10 57 I Part 1: Determining the Molar Enthalpy of Reaction 1. Graph the temperature of the reaction vs the time elapsed. Insert your graph in the space below (2 marks) 2. Calculate the molar enthalpy of reaction in KJ/mol NCH,0- using the data you have been provided for the experiment. (5 marks) Part 2 - Determining the Rate of Reaction 1. Use the data provided for part 2 to calculate the roles of reaction during the following reaction times: (2 marks x 3 questions 6 marks) 2. During 0 to 5 minutes lapsed b. During 5-10 minutes lapsed During 0-10 minutes lapsed 2. What happens to the reaction rate as the reaction progresses? Explain. (2 marks) Introduction: In the presence of water, citric acid (C,H,O,) and sodium bicarbonate (NaHCO3) (also known as baking soda) react to form trisodium citrate (Na,C,H,O;), water (H,0) and carbon dioxide (CO). C.M.Q. +3NaHCO, No,C,H,O, +31,01300, Vahiralarenereerible doen in 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


