Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hey can you do these easy MCQ questions for mE i dont need the working, just the answers Question 22 (1 point) If the standard
hey can you do these easy MCQ questions for mE 







i dont need the working, just the answers
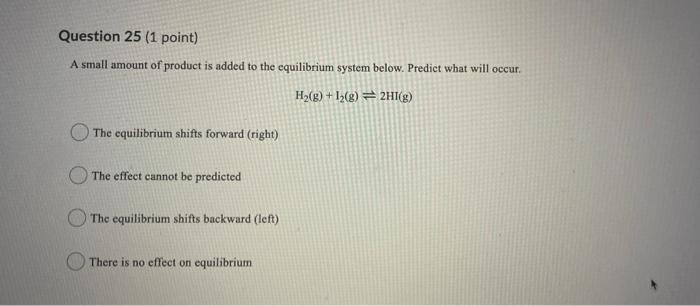
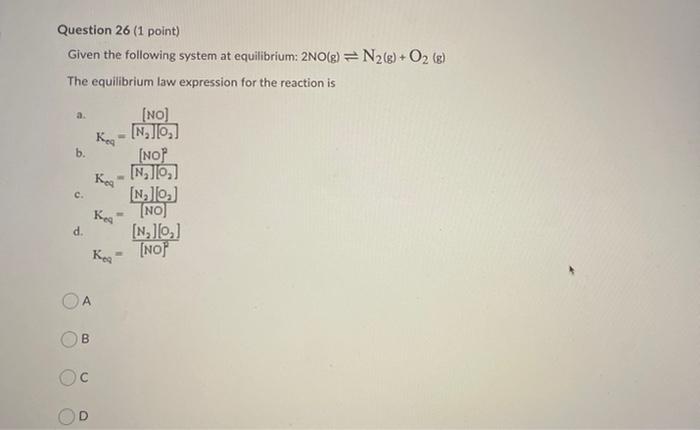
Question 22 (1 point) If the standard enthalpy of formation, Ah/, for Ag (aq) is +657 kJ/mol and for Cut (aq) is +105.8 kJ/mol what is the standard enthalpy of reaction, AH,, for the reaction below: 248) + Cu Cu +2Ag (29) (aq) +145.9 kJ/mol Cu -145.9 kJ/mol Cu -25.6 kJ/mol Cu +25.6 kJ/mol Cu Question 23 (1 point) If the standard enthalpy of formation, AH' for Ag (aq) is +65.7 kJ/mol and for Cu?" (aq) is +105.8 kJ/mol what is the standard enthalpy of reaction, AH,, for the reaction below: Culo) + 2Ag (24) 2A + Cu(aq) -25.6 kJ/mol Cu +145.9 kJ/mol Cu -145.9 kJ/mol Cu 125.6 kJ/mol Cu Question 24 (1 point) The volume of the system below is increased. Predict what will occur. H2(g) +12(8) 2HI(g) The equilibrium shifts forward (right) The equilibrium shifts backward (Ief) There is no effect on equilibrium The effect cannot be predicted Question 25 (1 point) A small amount of product is added to the equilibrium system below. Predict what will occur. H2(g) + 13(g) + 2H (8) The equilibrium shifts forward (right) The effect cannot be predicted The equilibrium shifts backward (left) There is no effect on equilibrium Question 26 (1 point) Given the following system at equilibrium: 2NO(g) = N2(g) + O2 (s) The equilibrium law expression for the reaction is a. b. (NO) Kea INJO [NOP INJO) NlO) [NO] [N, JO,] [NO] Keg c. Kreat d. 00 B D Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


