Hi, can you help me out? I'm not sure if the answers are right. Thank you
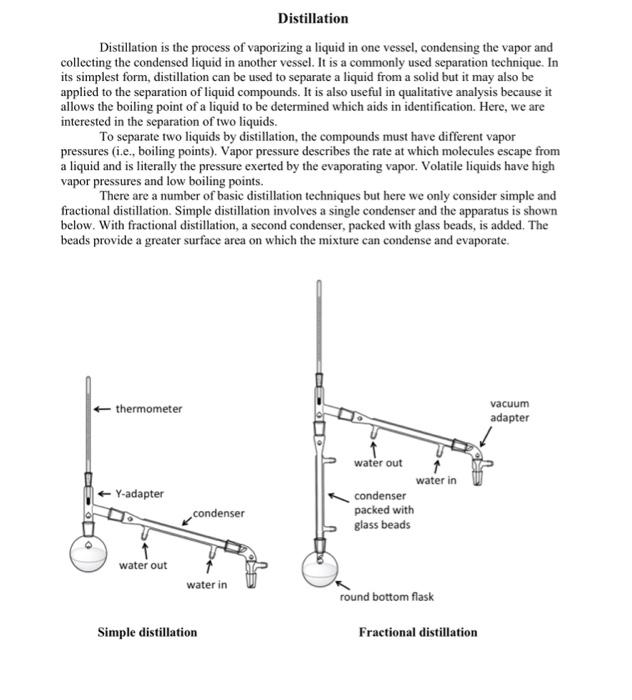
Distillation Distillation is the process of vaporizing a liquid in one vessel, condensing the vapor and collecting the condensed liquid in another vessel. It is a commonly used separation technique. In its simplest form, distillation can be used to separate a liquid from a solid but it may also be applied to the separation of liquid compounds. It is also useful in qualitative analysis because it allows the boiling point of a liquid to be determined which aids in identification. Here, we are interested in the separation of two liquids. To separate two liquids by distillation, the compounds must have different vapor pressures (i.e., boiling points). Vapor pressure describes the rate at which molecules escape from a liquid and is literally the pressure exerted by the evaporating vapor. Volatile liquids have high vapor pressures and low boiling points. There are a number of basic distillation techniques but here we only consider simple and fractional distillation. Simple distillation involves a single condenser and the apparatus is shown below. With fractional distillation, a second condenser, packed with glass beads, is added. The beads provide a greater surface area on which the mixture can condense and evaporate. Distillation Procedure 1. Place 30ml of an unknown liquid mixture in a 100ml round bottom flask. Add a boiling chip. The mixture consists of 50% unknown A and 50% of unknown B. You will separate these unknowns by distillation and determine their boiling point. 2. Set up the distillation apparatus as shown in the figure. If you have two condensers at your bench and one is packed with glass beads, you are performing the fractionation distillation. If you only have one unpacked condenser, you are performing the simple distillation. Attach tubing to the outlets of the unpacked condenser. Use your finger to wet the joint with water where you are placing the tubing, and twist it to attach. 3. Support the heating mantle with a ring clamp and build the distillation apparatus from the base up. Make sure the ring clamp is positioned 10cm above the surface of the bench top so that you can lower it, removing the source of heat if necessary. Use a small amount of stopeock grease on all joints. Apply a bead of grease to one surface, assemble the joint, and then rotate the joint to spread the grease over both surfaces. The joint should turn from opaque to translucent. Use keck clips to hold joints together. Use clamps to hold the distilling flask and condenser/s in place. Clamp loosely. They are for support, not to hold the apparatus together. Look at the example in the lab for placement of clamps. One should be on the distilling pot and one on the cold condenser. If you are preforming fractional distillation, a clamp is also required on the packed condenser. 4. Make sure that the bulb of the thermometer is just below the arm of the Y-adapter. Also ensure that the bottom of the round bottom flask (the distilling pot) is touching the heating surface of the mantel. Plug the mantle into the power controller (NOT directly into the outlet) and plug the power controller into the outlet. 5. Attach the tubing on the condenser to the cold-water tap. Note which outlet is 'in' and 'out' and place the 'out' tubing in the sink. Connect the other end of the "in" tubing to the cold-water tap. Water always enters the condenser so that the coldest water is furthest away from the hot vapour. 6. Have your professor or TA check the distillation apparatus. 7. Gradually turn on the tap so that there is a low flow through the tubing and condenser. You do not need a high water pressure. High flow rates generate high pressure and increase the chances of the tubing popping of the condenser. Turn on the power to the heating mantle and begin heating. You should have a distillation rate of about one drop every 3 or 4 seconds. If the rate is faster than that, turn the power controller to a lower setting. A setting of 60 is usually sufficient for the simple distillation. A setting of 70 should be used with the fractional distillation. If the rate slows or the mixture stops boiling, increase the setting on the controller. 8. Be prepared to collect the distillate in a 10mL graduated cylinder. Begin recording temperatures when the first drop of distillate is collected. After the initial drop, record the temperature for every 1mL of distillate collected. You will have to empty the graduated cylinder several times. Continue to distill until only a few mL remain in the distilling pot (so that the boiling chip is just covered). When the distillation is finished, lower the heating mantle to remove the heat source and let the apparatus cool. NEVER let the distilling pot go dry. Place all solvents in the waste beakers at the end of the benches. Do not pour any solvents into the sink. 9. Exchange your temperature and volume data with a neighbor at your bench who used the distillation apparatus that you did not. What best describes the condenser used in this lab? a) a glass tube with a glass sleeve around it that water flows through b) a glass tube with many smaller tubes through which the solvent mixture flows c) a glass tube where water mixes with solvents during distillation What's the purpose of water in this distillation? a) creates a vacuum so that the solvent mixture boils at a lower temperature b) cools the condenser c) mixes with solvents to help separation d) all of the above How are the ground glass joints held together during the distillation? a) Keck clips b) rubber bands c) tape d) stopcock grease e) clamps













