Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hi can you help me work out these problem step by step I ONLY NEED HELP WITH QUESTIOS NUMBER 5,6,7,8 i attach the answer for
hi can you help me work out these problem step by step 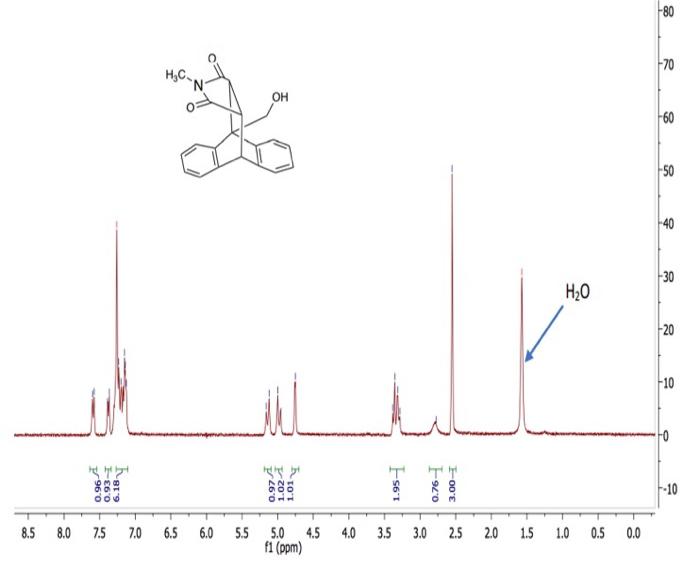

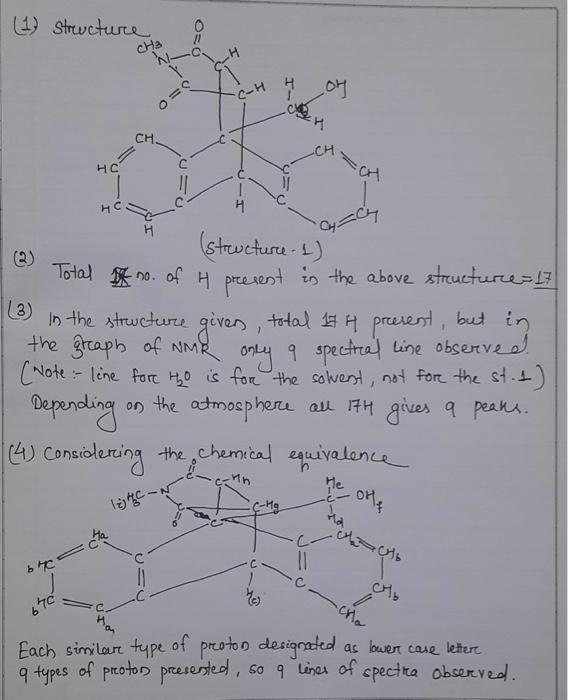
1. Draw in all hydrogen atoms in the chemical structure. Remember that there are always four bonds to carbon. 2. How many hydrogen atoms are in the structure? 3. The total number of hydrogen atoms in the chemical structure should equal the total number of protons integrated in the spectrum. What is the total number of protons integrated in the spectrum? Does the total number of hydrogen atoms in the structure match the total number of protons integrated in the spectrum? Show your work. 4. Consider the chemical equivalence (number of different kinds of protons) within the molecule. Label each different type of proton with a lower-case letter. How many signals would be expected to appear in the spectrum? Hint, the number of signals that should be in the spectrum may be overlapping in the true spectrum. Diastereotopic protons are not equivalent. 5. What are diastereotopic protons? Which two protons in this molecule are diastereotopic? 6. Use the 'H NMR Chemical Shift Table to predict the ppm range expected for each chemically equivalent proton listed in Problem 4. 7. Predict the splitting pattern of each proton signal. Splitting patterns are predicted by identifying the number of equivalent neighboring protons (singlet, doublet, triplet, quartet, multiplet...) ( 8. Discuss how the 1H NMR spectrum can be used to help prove the identity of the product. Consider the structural features (chemical shifts and presence or absence of signals) and the number of protons (integration) in the product compared to the starting material. ( (2) Total no. of H present in the above structure =17 (3) In the structure given, total 14H present, but in the graph of NMR only 9 spectral line observe d. (vote:- line for H2O is for the solvent, not for the st. 1 ) Depending on the atmosphere all 17H gives 9 peaks. (4) Considering the chemical equivalence Each similar type of proton designated as bwer case letter q types of proton presented, so q lines of spectica observed. 1. Draw in all hydrogen atoms in the chemical structure. Remember that there are always four bonds to carbon. 2. How many hydrogen atoms are in the structure? 3. The total number of hydrogen atoms in the chemical structure should equal the total number of protons integrated in the spectrum. What is the total number of protons integrated in the spectrum? Does the total number of hydrogen atoms in the structure match the total number of protons integrated in the spectrum? Show your work. 4. Consider the chemical equivalence (number of different kinds of protons) within the molecule. Label each different type of proton with a lower-case letter. How many signals would be expected to appear in the spectrum? Hint, the number of signals that should be in the spectrum may be overlapping in the true spectrum. Diastereotopic protons are not equivalent. 5. What are diastereotopic protons? Which two protons in this molecule are diastereotopic? 6. Use the 'H NMR Chemical Shift Table to predict the ppm range expected for each chemically equivalent proton listed in Problem 4. 7. Predict the splitting pattern of each proton signal. Splitting patterns are predicted by identifying the number of equivalent neighboring protons (singlet, doublet, triplet, quartet, multiplet...) ( 8. Discuss how the 1H NMR spectrum can be used to help prove the identity of the product. Consider the structural features (chemical shifts and presence or absence of signals) and the number of protons (integration) in the product compared to the starting material. ( (2) Total no. of H present in the above structure =17 (3) In the structure given, total 14H present, but in the graph of NMR only 9 spectral line observe d. (vote:- line for H2O is for the solvent, not for the st. 1 ) Depending on the atmosphere all 17H gives 9 peaks. (4) Considering the chemical equivalence Each similar type of proton designated as bwer case letter q types of proton presented, so q lines of spectica observed I ONLY NEED HELP WITH QUESTIOS NUMBER 5,6,7,8
i attach the answer for the first three just to help you out not waste time
thanks! i really needed by today since our lab is today



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


