Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi could I get help with these thanks! The formation of methanol (CH3OH) is important to the processing of new fuels. At 25.C,Kp= 2.25104 for
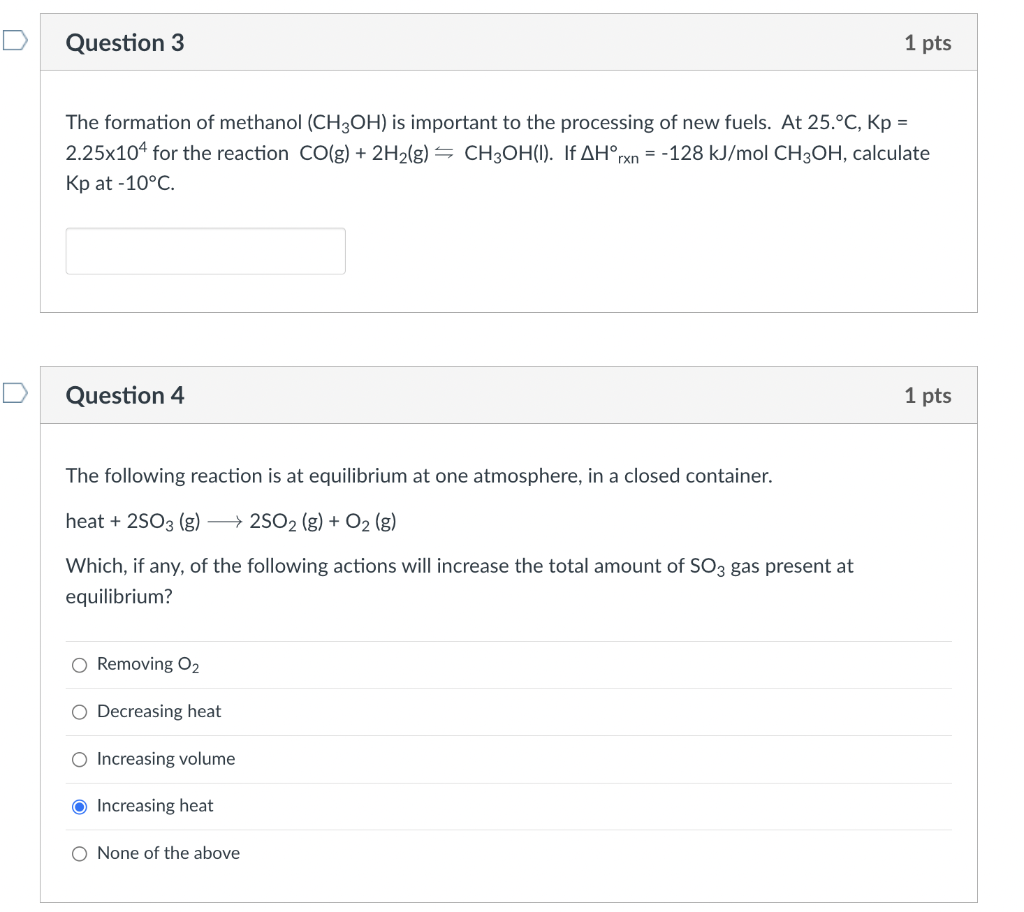
Hi could I get help with these thanks!
The formation of methanol (CH3OH) is important to the processing of new fuels. At 25.C,Kp= 2.25104 for the reaction CO(g)+2H2(g)CH3OH(l). If Hrxn=128kJ/molCH Kp at 10C. Question 4 The following reaction is at equilibrium at one atmosphere, in a closed container. heat +2SO3(g)2SO2(g)+O2(g) Which, if any, of the following actions will increase the total amount of SO3 gas present at equilibrium? Removing O2 Decreasing heat Increasing volume Increasing heat None of the aboveStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


