Answered step by step
Verified Expert Solution
Question
1 Approved Answer
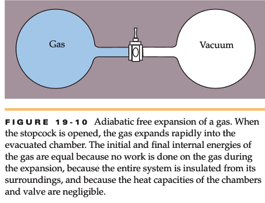
Hi, could I get some help with this macro-connection physics problem involving adiabatic free expansion and entropy? The set up is: For an isothermal reversible
Hi, could I get some help with this macro-connection physics problem involving adiabatic free expansion and entropy?
The set up is:
For an isothermal reversible expansion of two moles of an ideal gas with adiabatic free expansion in a rigid container as in the picture, what is the entropy change of the a) gas (if the volume only doubles instead of quadruples) and b) the surroundings in J/K to 4 digits of precision, assuming NA = 6.022e23 and kB = 1.38e-23 J/K)?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


