hi everyone
would you please help me answering these questions, its due in 2 hours.
thanks
Complete the following scheme by adding the missing parts: (7 answers) : acetic acid White precipitate (B)--- Yellow precipitate ---------- -D- NH,Cl (solid) + NH,OH (sol.) + (NH),Co, (solid) potassium chromate solution salt solution ---(A)-- Flame test Calcium sulphate The cation is (G)- The color of flame change to -(F)- White precipitate --(C)----- G=Barium (ll) cation a A= Calcium (1) chloride .bO C=Barium sulphate.co B=Barium (1) bicarbonated A= Barium (II) chloride .eo B=Bariurn sulphate .fo A= Copper (II) chloride 80 C=Barium thiosulphate ho G= Copper (II) cation 10 B B-Barium carbonate jo D- Barium sulphate .kO C=Barium sulphite 10 F. Yellowish green.m Gm Bismuth (Ill) cation .no Dr Barium chromato .00 DE Barium hydroxide po E Soluble .40 E=Insoluble ro E= Dissolve so F= Apple green to F = Orange to redu
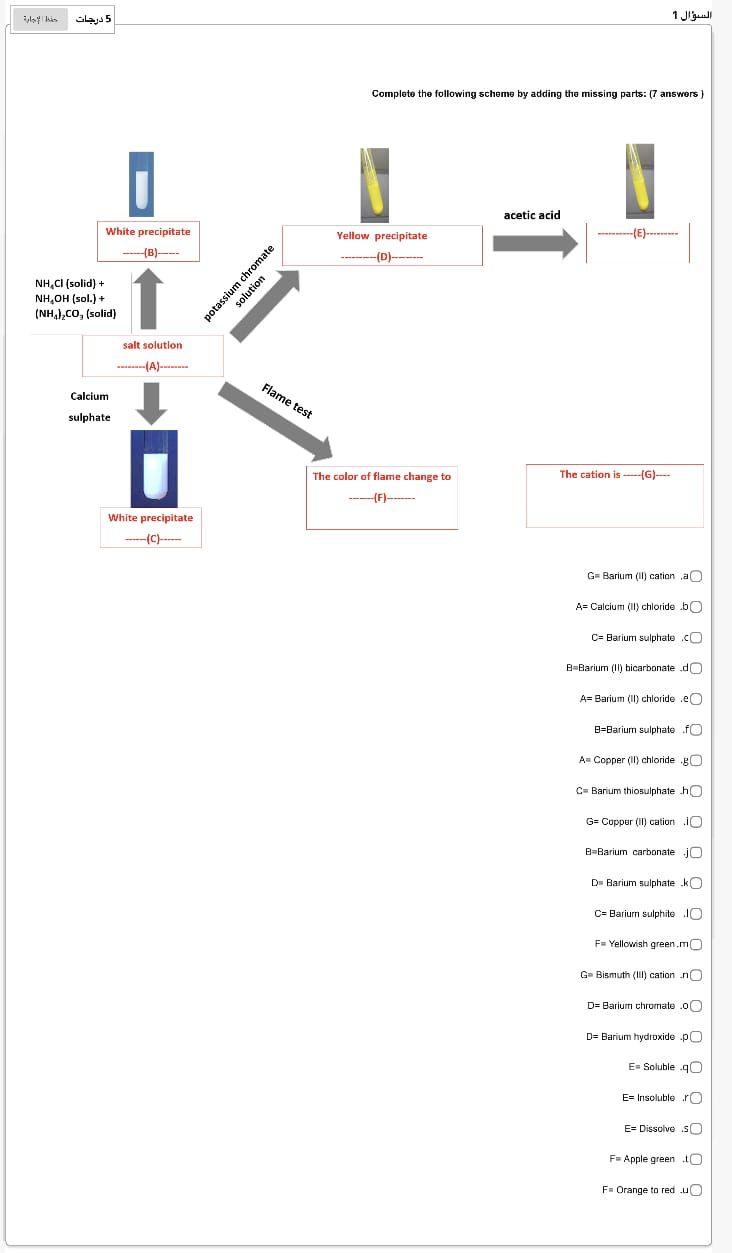
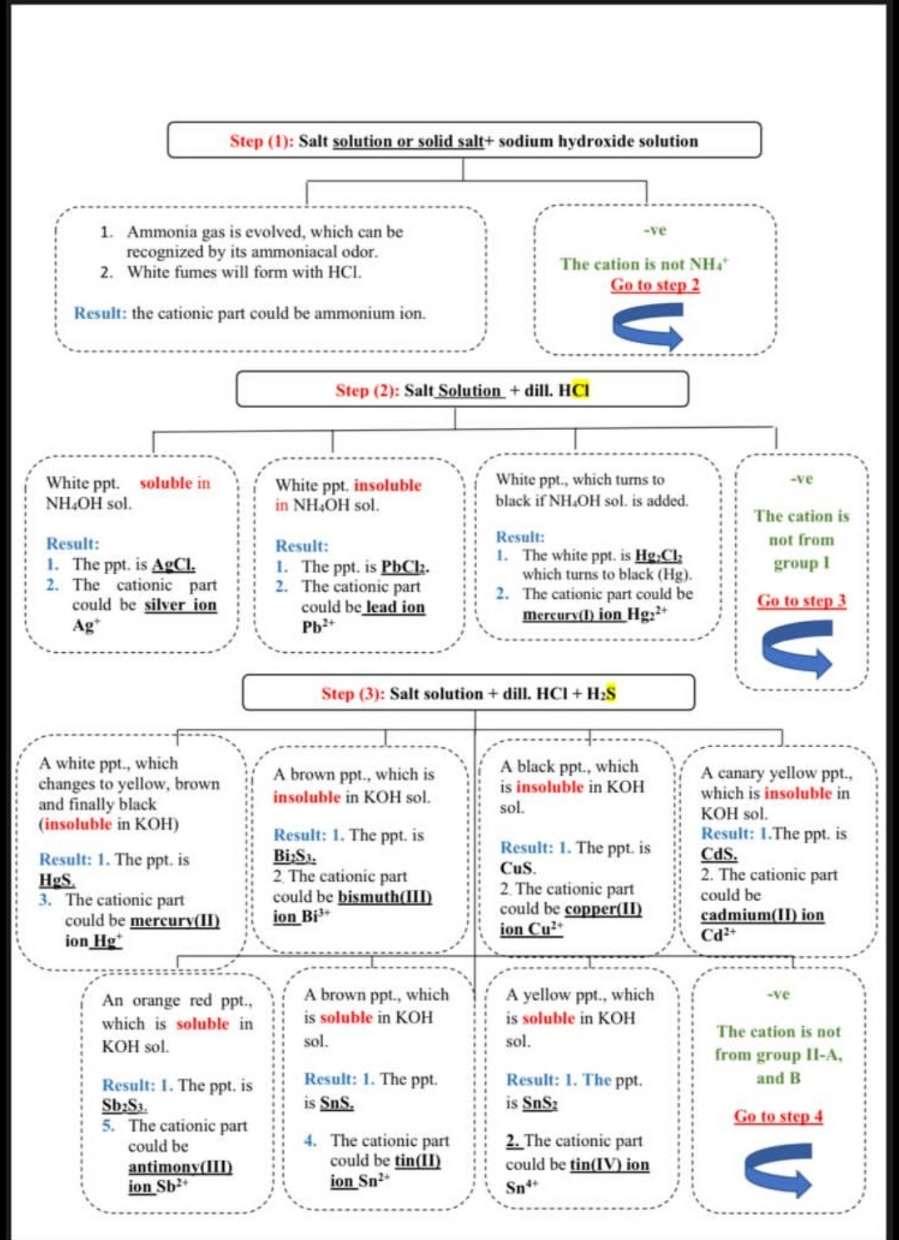
Sloy his 5 1 Complete the following scheme by adding the missing parts: (7 answers) : acetic acid White precipitate (B)--- Yellow precipitate ----------- NH,Cl (solid) + NH,OH (sol. + (NH),Co, (solid) potassium chromate solution salt solution ---- Flame test Calcium sulphate The cation is (G)- The color of flame change to --(F)- White precipitate --(C)----- G=Barium (ll) cational A= Calcium (1) chlorido bO C=Barium sulphate.co B-Barium (11) bicarbonate do A= Barium (II) chloride .eo B=Bariurn sulphate fo A Copper (II) chloride go C=Barium thiosulphate ho G= Copper (II) cation 10 B B=Barium carbonate jo D- Barium sulphate .kO C=Barium sulphite 10 F- Yellowish green.mo Ga Bismuth (Ill) cation.no Dr Barium chromato .o0 D= Barium hydroxide .pO E Soluble 40 E= Insoluble ro E= Dissolve so F= Apple green 10 F = Orange to redu Step (1): Salt solution or solid salt+ sodium hydroxide solution 1. Ammonia gas is evolved, which can be recognized by its ammoniacal odor. 2. White fumes will form with HCL. JO The cation is not NHA Go to step 2 Result: the cationic part could be ammonium ion. U Step (2): Salt Solution + dill. HCI -ve White ppt. soluble in NH4OH sol. White ppt, insoluble in NH OH sol. White ppt., which turns to black if NH.OH sol. is added. The cation is not from group 1 Result: 1. The ppt is AgCI. 2. The cationic part could be silver son Ag Result: 1. The ppt. is PbCl2. 2. The cationic part could be lead ion Pb? Result: 1. The white ppt is Hg.Cl: which turns to black (Hg). 2. The cationic part could be Mercury(t) ion Hg2+ Go to step 3 G Step (3): Salt solution + dill. HCI + H2S A white ppt., which changes to yellow, brown and finally black (insoluble in KOH) A brown ppt, which is insoluble in KOH sol. A black ppt., which is insoluble in KOH sol. Result: 1. The ppt.is HgS. 3. The cationic part could be mercury(II) ion Hg Result: 1. The ppt.is Bi:Si. 2 The cationic part could be bismuth(III) ion Bi? Result: 1. The ppt.is Cus. 2 The cationic part could be copper(II) ion Cul A canary yellow ppt. which is insoluble in KOH sol. Result: 1. The ppt.is Cds. 2. The cationic part could be cadmium(IT) ion Cd? -ve An orange red ppt.. which is soluble in KOH sol. A brown ppt., which is soluble in KOH A yellow ppt, which is soluble in KOH sol. sol. The eation is not from group II-A, and B Result: 1. The ppt is Sns. Result: 1. The ppt. is SnS: Go to step 4 Result: 1. The ppt.is SbSi. 5. The cationic part could be antimony(III) 4. The cationic part could be tin(II) ion Sn? 2. The cationic part could be tin(IV) ion Sn" U ion Sb








