Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi, I need help with the last 2 questions below. Please show steps and include units. Thank you Chemistry food dye concentration Dilution formula calculation
Hi, I need help with the last 2 questions below. Please show steps and include units. Thank you
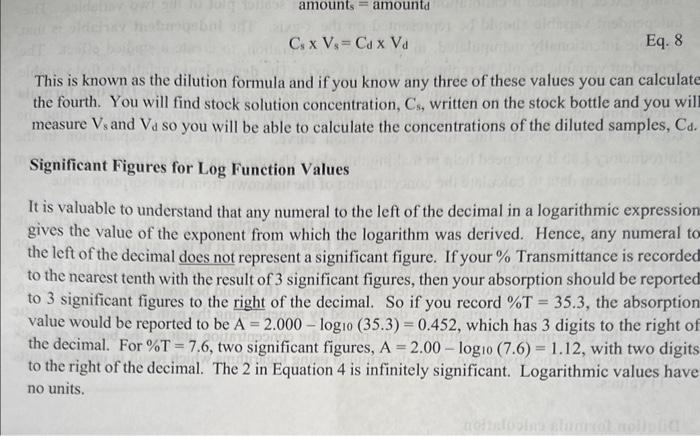
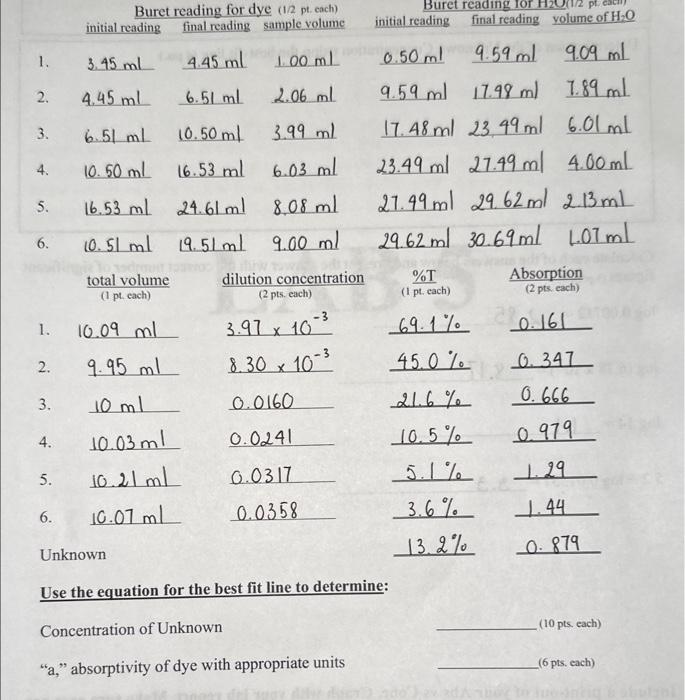
Dilution formula calculation To calculate the concentrations of the diluted samples, remember that the amount of a solute in a diluted sample is equal to the amount of solute in the original aliquot (sample removed from a stock solution). Diluting it with a solvent does NOT change the amount of solute. You can find the amount of solute in a sample of known concentration if you multiply its concentration by its volume (Equation 6). (We will generically describe concentration as amount per volume (amount/volume) where, for example, amount = mole if the molarity of the sample is known or amount = grams if the concentration is known in gram/volume.) amounts=CsVs Eq. 6 where amounts is the initial amount of solute in the aliquot, Cs is the initial concentration of stock solution and Vs is the volume of the stock solution aliquot. The amount in the diluted solution is given by a similar equation (Equation 7). amounttd=CdVd Eq. 7 where amount is the amount of solute in the diluted solution ("final" amount), Cd is the new concentration, and Vd is the total final volume of the diluted sample. Since, as just indicated, diluting the solution does not change the amount of solute, CsVs=CdVd Eq. 8 This is known as the dilution formula and if you know any three of these values you can calculate the fourth. You will find stock solution concentration, C5, written on the stock bottle and you wil measure Vs and Vd so you will be able to calculate the concentrations of the diluted samples, Cd. Significant Figures for Log Function Values It is valuable to understand that any numeral to the left of the decimal in a logarithmic expressior gives the value of the exponent from which the logarithm was derived. Hence, any numeral t the left of the decimal does not represent a significant figure. If your % Transmittance is recordec to the nearest tenth with the result of 3 significant figures, then your absorption should be reportec to 3 significant figures to the right of the decimal. So if you record %T=35.3, the absorption value would be reported to be A=2.000log10(35.3)=0.452, which has 3 digits to the right of the decimal. For %T=7.6, two significant figures, A=2.00log10(7.6)=1.12, with two digits to the right of the decimal. The 2 in Equation 4 is infinitely significant. Logarithmic values have no units. Use the equation for the best fit line to determine: Concentration of Unknown (10 pts. each) "a," absorptivity of dye with appropriate units (6 pts. each) Chemistry food dye concentration 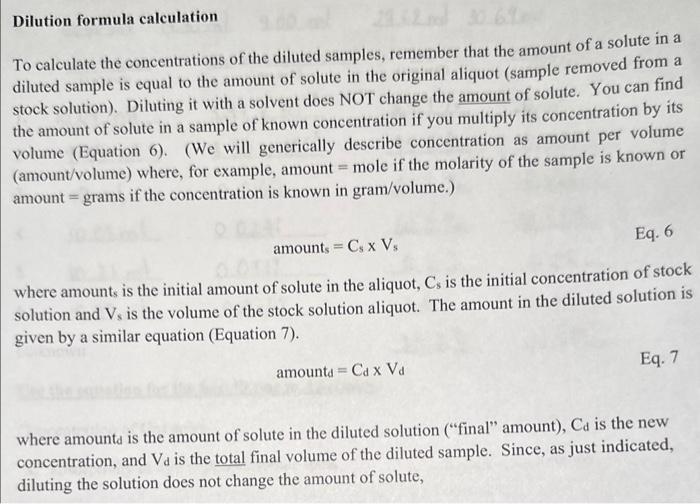



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


