Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi, i posted this question 4 times and didnt get a correct answer. Please dont upload the same one as before and answer the questions,
Hi, i posted this question 4 times and didnt get a correct answer. Please dont upload the same one as before and answer the questions, especially a.
Gas A is decomposed at 700 C with a partial pressure of 1 atm, with a first-order irreversible reaction, in a constant bed isothermal reactor, volume 100 cm3. The reactor contains spherical catalyst granules, 5 mm in diameter, and the bed porosity is 0.5. The rate of decomposition is 0.25 Kmol/ sec. The effective diffusion of the reactant in the catalyst granules is 1.0 x 10-6 m2 1/ sec. a)Develop the equation of external isothermal and non-isothermal efficiency factor for a zero order reaction.(A->B) b) Write the thiele modulus expression as well as the efficiency factor and then calculate them right(with right units). c) What can I do to eliminate all resistances of internal diffusion and calculate the radius to achieve that thanks and i want it from an expert who knows what he is doing. DONT upload the same one again. 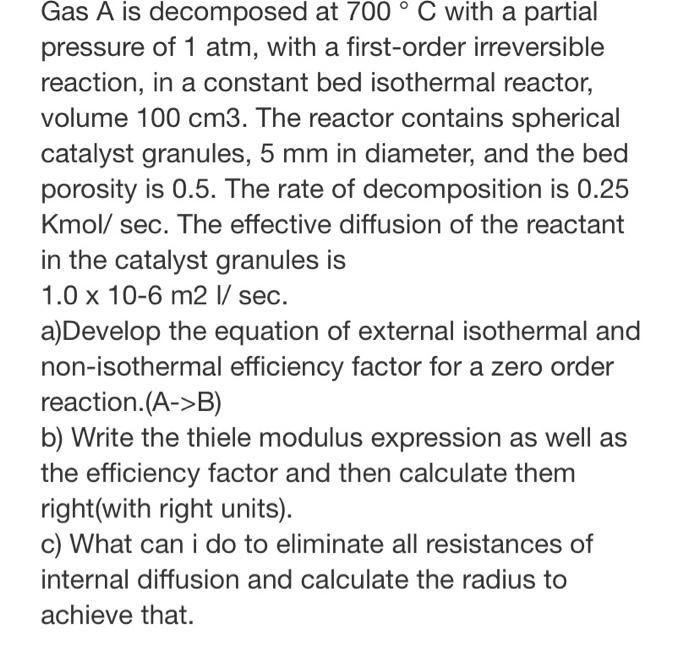

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


