Hi,
I was working on my hwk, but would like to double check my answer. Could you please solve it?
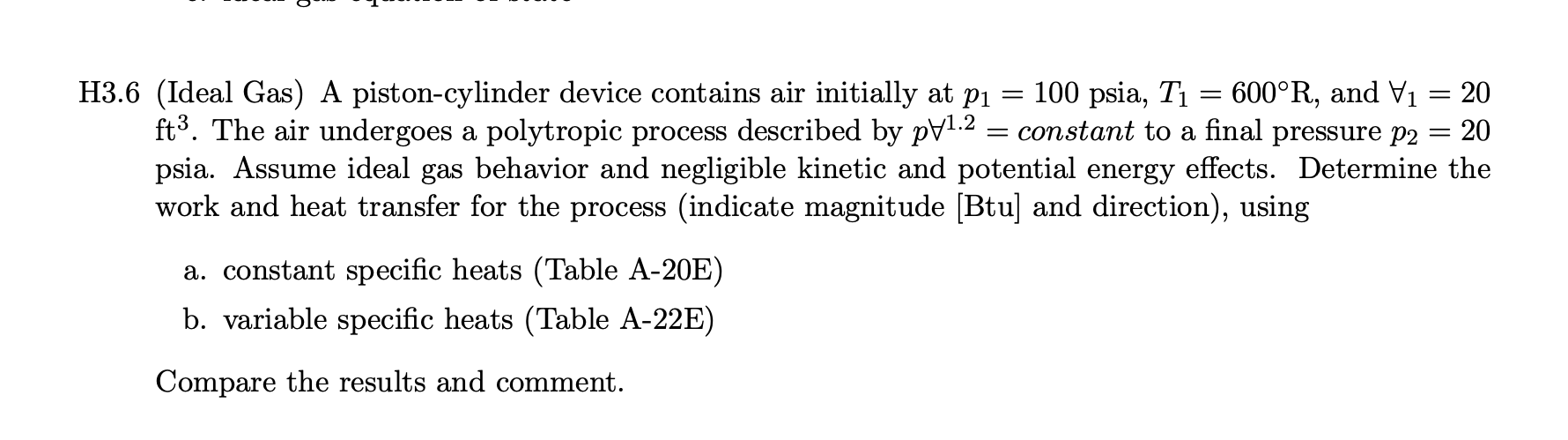
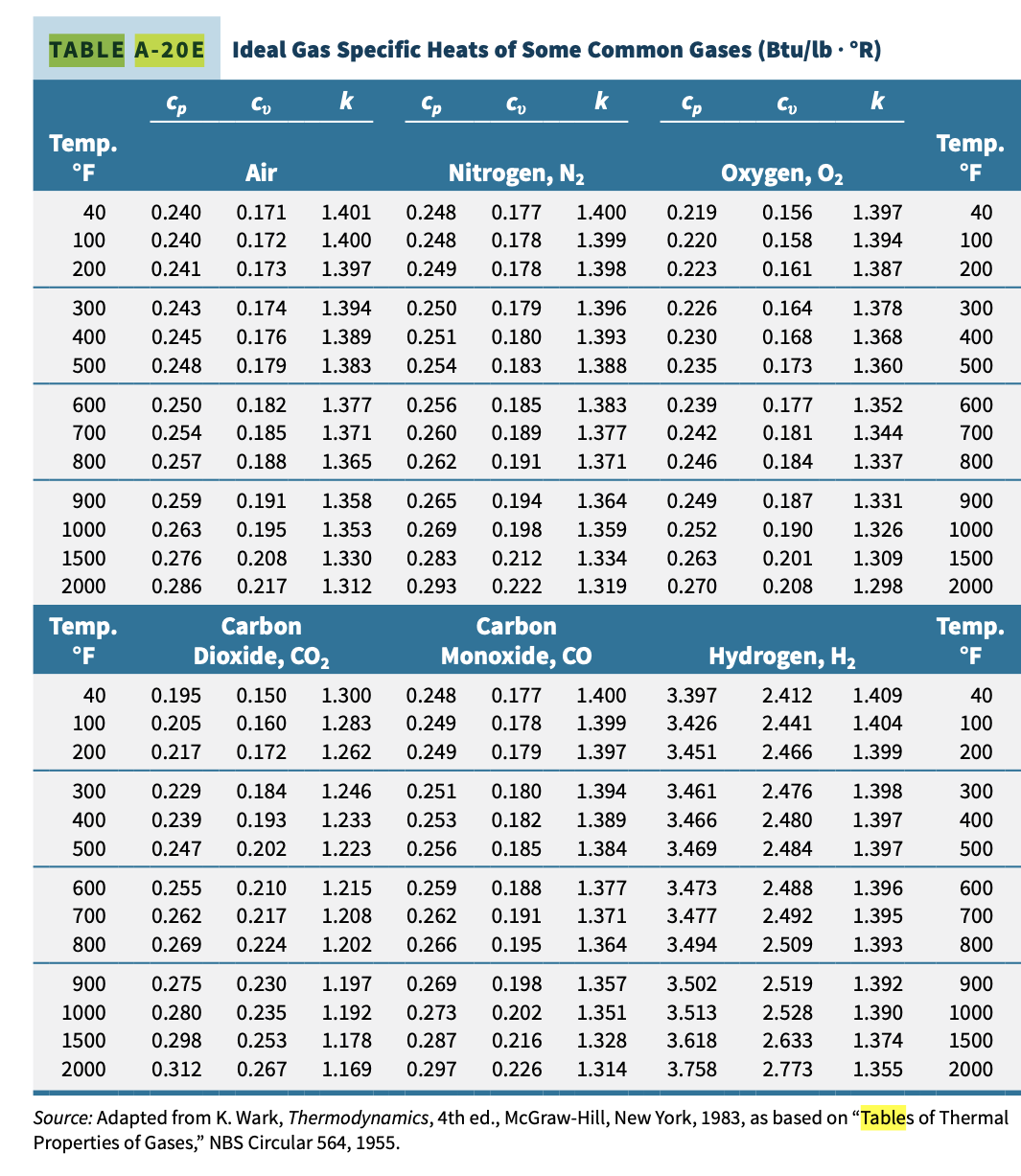
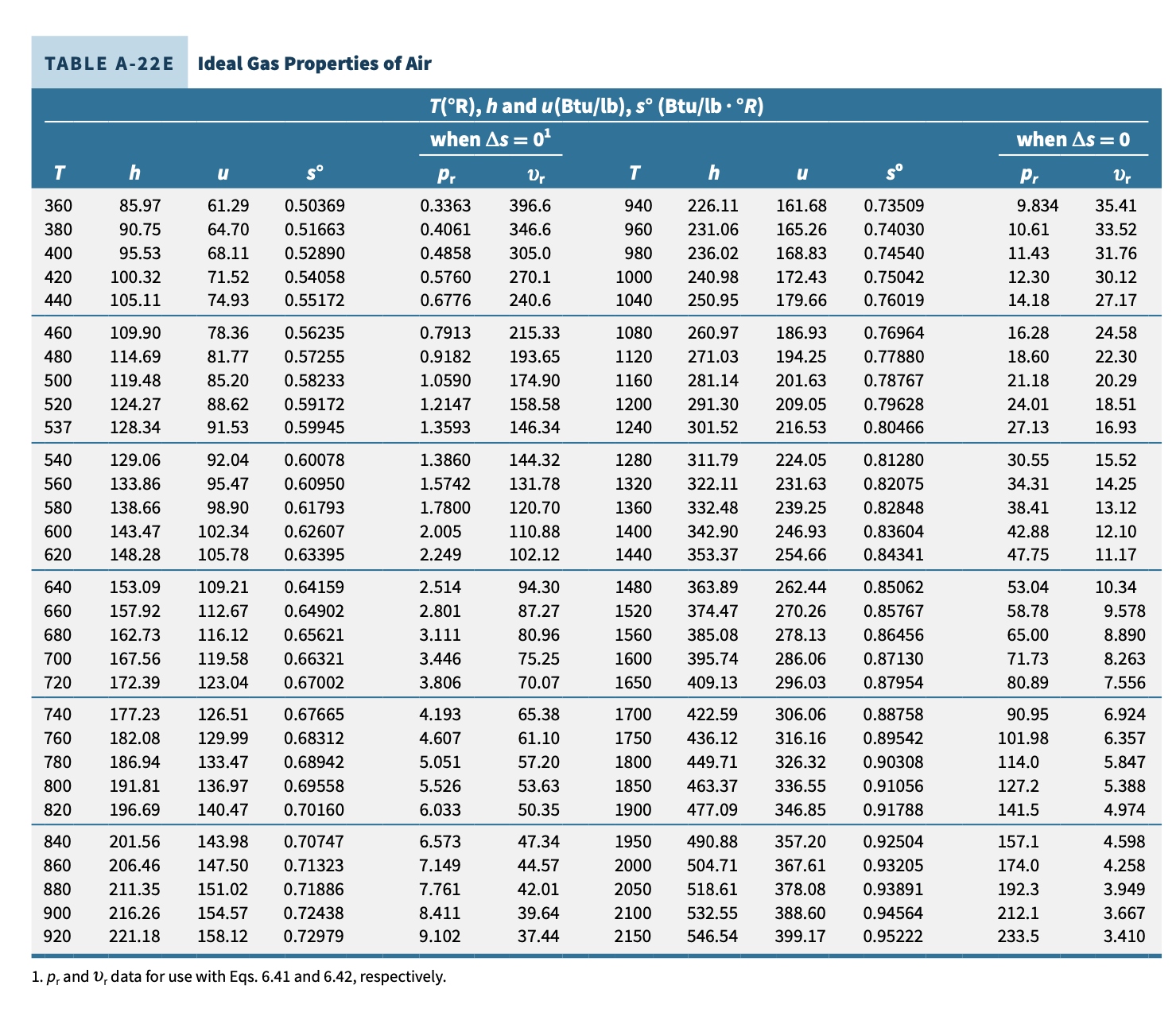
H3.6 (Ideal Gas) A piston-cylinder device contains air initially at p1 = 100 psia, T1 = 600 R, and V1 = 20 fts. The air undergoes a polytropic process described by pV1 2 = constant to a final pressure p2 = 20 psia. Assume ideal gas behavior and negligible kinetic and potential energy effects. Determine the work and heat transfer for the process (indicate magnitude [Btu] and direction), using a. constant specific heats (Table A-20E) b. variable specific heats (Table A-22E) Compare the results and comment.TABLE A-20E Ideal Gas Specific Heats of Some Common Gases (Btu/lb . 'R) Cp k Cp k Cp k Temp. Temp. OF Air Nitrogen, N2 Oxygen, 02 40 0.240 0.171 1.401 0.248 0.177 1.400 0.219 0.156 1.397 40 100 0.240 0.172 1.400 0.248 0.178 1.399 0.220 0.158 1.394 100 200 0.241 0.173 1.397 0.249 0.178 1.398 0.223 0.161 1.387 200 300 0.243 0.174 1.394 0.250 0.179 1.396 0.226 0.164 1.378 300 400 0.245 0.176 1.389 0.251 0.180 1.393 0.230 0.168 1.368 400 500 0.248 0.179 1.383 0.254 0.183 1.388 0.235 0.173 1.360 500 600 0.250 0.182 1.377 0.256 0.185 1.383 0.239 0.177 1.352 600 700 0.254 0.185 1.371 0.260 0.189 1.377 0.242 0.181 1.344 700 800 0.257 0.188 1.365 0.262 0.191 1.371 0.246 0.184 1.337 800 900 0.259 0.191 1.358 0.265 0.194 1.364 0.249 0.187 1.331 900 1000 0.263 0.195 1.353 0.269 0.198 1.359 0.252 0.190 1.326 1000 1500 0.276 0.208 1.330 0.283 0.212 1.334 0.263 0.201 1.309 1500 2000 0.286 0.217 1.312 0.293 0.222 1.319 0.270 0.208 1.298 2000 Temp. Carbon Carbon Temp. OF Dioxide, CO2 Monoxide, CO Hydrogen, H2 PF 40 0.195 0.150 1.300 0.248 0.177 1.400 3.397 2.412 1.409 40 100 0.205 0.160 1.283 0.249 0.178 1.399 3.426 2.441 1.404 100 200 0.217 0.172 1.262 0.249 0.179 1.397 3.451 2.466 1.399 200 300 0.229 0.184 1.246 0.251 0.180 1.394 3.461 2.476 1.398 300 400 0.239 0.193 1.233 0.253 0.182 1.389 3.466 2.480 1.397 400 500 0.247 0.202 1.223 0.256 0.185 1.384 3.469 2.484 1.397 500 600 0.255 0.210 1.215 0.259 0.188 1.377 3.473 2.488 1.396 600 700 0.262 0.217 1.208 0.262 0.191 1.371 3.477 2.492 1.395 700 800 0.269 0.224 1.202 0.266 0.195 1.364 3.494 2.509 1.393 300 900 0.275 0.230 1.197 0.269 0.198 1.357 3.502 2.519 1.392 900 1000 0.280 0.235 1.192 0.273 0.202 1.351 3.513 2.528 1.390 1000 1500 0.298 0.253 1.178 0.287 0.216 1.328 3.618 2.633 1.374 1500 2000 0.312 0.267 1.169 0.297 0.226 1.314 3.758 2.773 1.355 2000 Source: Adapted from K. Wark, Thermodynamics, 4th ed., McGraw-Hill, New York, 1983, as based on "Tables of Thermal Properties of Gases," NBS Circular 564, 1955.TABLE A-22E Ideal Gas Properties of Air T(R), h and u(Btu/lb), so (Btu/lb . R) when As = 01 when As = 0 T h So Pr Ur h So Pr Ur 161.68 0.73509 9.834 35.41 360 85.97 61.29 0.50369 0.3363 396.6 940 226.11 380 90.75 64.70 0.51663 0.4061 346.6 960 231.06 165.26 0.74030 10.61 33.52 95.53 68.11 0.52890 0.4858 305.0 980 236.02 168.83 0.74540 11.43 31.76 400 30.12 420 100.32 71.52 0.54058 0.5760 270.1 1000 240.98 172.43 0.75042 12.30 440 105.11 74.93 0.55172 0.6776 240.6 1040 250.95 179.66 0.76019 14.1 27.17 215.33 1080 260.97 186.93 0.76964 16.28 24.58 460 109.90 78.36 0.56235 0.7913 114.69 81.77 0.57255 0.9182 193.65 1120 271.03 194.25 0.77880 18.60 22.30 480 500 119.48 85.20 0.58233 1.0590 174.90 1160 281.14 201.63 0.78767 21.18 20.29 520 124.27 88.62 0.59172 1.2147 158.58 1200 291.30 209.05 0.79628 24.01 18.51 537 128.34 91.53 0.59945 1.3593 146.34 1240 301.52 216.53 .80466 27.1 16.93 1.3860 144.32 1280 311.79 224.05 0.81280 30.55 15.52 540 129.06 92.04 0.60078 14.25 560 133.86 95.47 0.60950 1.5742 131.78 322.11 231.63 0.82075 34.31 580 138.66 98.90 0.61793 1.7800 120.70 1360 332.48 239.25 0.82848 38.41 13.12 42.88 12.10 600 143.47 102.34 0.62607 2.00 110.88 1400 342.90 246.93 0.83604 148.28 105.78 0.63395 2.249 102.12 1440 353.37 254.66 0.84341 47.75 11.17 620 640 153.09 109.21 0.64159 2.514 94.30 1480 363.89 262.44 0.85062 53.04 10.34 560 157.92 112.67 0.64902 2.801 87.27 .520 374.47 270.26 0.85767 58.78 9.578 0.86456 65.00 8.890 680 162.73 116.12 0.65621 3.111 80.96 1560 385.08 278.13 700 167.56 119.58 0.66321 3.446 75.25 1600 395.74 286.06 0.87130 71.73 8.263 720 172.39 123.04 0.67002 3.806 70.07 1650 409.13 296.03 0.87954 80.8 7.556 4.193 65.38 1700 422.59 306.06 0.88758 90.95 6.924 740 177.23 126.51 0.67665 760 182.08 129.99 0.68312 4.607 61.10 .750 436.12 316.16 0.89542 101.98 6.357 0.68942 5.051 57.20 1800 449.71 326.32 0.90308 114.0 5.847 780 186.94 133.47 127.2 5.388 800 191.81 136.97 0.69558 5.526 53.63 1850 463.37 336.55 0.91056 6.033 1900 477.09 346.85 141.5 320 196.69 140.47 0.70160 50.35 0.91788 4.974 6.573 47.34 1950 490.88 357.20 0.92504 157.1 4.598 840 201.56 143.98 0.70747 4.258 860 206.46 147.50 0.71323 7.149 44.57 2000 504.71 367.61 0.93205 174.0 211.35 151.02 0.71886 7.761 42.01 2050 518.61 378.08 0.93891 192.3 3.949 380 900 216.26 154.57 0.72438 8.411 39.64 2100 532.55 388.60 0.94564 212.1 3.667 399.17 320 221.18 158.12 0.72979 9.102 37.44 2150 546.54 0.95222 233.5 3.410 1. p, and , data for use with Eqs. 6.41 and 6.42, respectively.TABLE A-22E Ideal Gas Properties of Air (Continued) T(R), h and u (Btu/lb), so (Btu/lb . R) when As = 01 when As = 0 Pr Ur T h U So Pr Ur 2200 560.59 409.78 0.95868 256.6 3.176 3700 998.11 744.48 1.10991 2330 0.5882 2250 574.69 420.46 0.96501 281.4 2.961 3750 1013.1 756.04 1.11393 2471 0.5621 2300 588.82 431.16 0.97123 308.1 2.765 3800 1028.1 767.60 1.11791 2618 0.5376 235 603.00 441.91 0.97732 336.8 2.585 3850 1043.1 779.19 1.12183 2773 0.5143 240 617.22 452.70 0.98331 367.6 2.419 3900 1058.1 790.80 1.12571 2934 0.4923 2450 631.48 463.54 0.98919 400.5 2.266 3950 1073.2 802.43 1.12955 3103 0.4715 2500 645.78 474.40 0.99497 435.7 2.125 4000 1088.3 814.06 1.13334 328 0.4518 2550 660.12 485.31 1.00064 473.3 1.996 4050 1103.4 825.72 1.13709 3464 0.4331 2600 674.49 496.26 1.00623 513.5 1.876 4100 1118.5 837.40 1.14079 3656 0.4154 2650 688.90 507.25 1.01172 556.3 1.765 4150 1133.6 849.09 1.14446 3858 0.3985 2700 703.35 518.26 1.01712 601.9 1.662 4200 1148.7 860.81 1.14809 4067 0.3826 2750 717.83 529.31 1.02244 650.4 1.566 4300 1179.0 884.28 1.15522 4513 0.3529 2800 732.33 540.40 1.02767 702.0 1.478 4400 1209.4 907.81 1.16221 4997 0.3262 2850 746.88 551.52 1.03282 756.7 1.395 4500 1239.9 931.39 1.16905 5521 0.3019 2900 761.45 562.66 1.03788 814.8 1.318 4600 1270.4 955.04 1.17575 6089 0.2799 2950 776.05 573.84 1.04288 876.4 1.247 4700 1300.9 978.73 1.18232 6701 0.2598 3000 790.68 585.04 1.04779 941.4 1.180 4800 1331.5 1002.5 1.18876 7362 0.2415 3050 805.34 596.28 1.05264 1011 1.118 4900 1362.2 1026.3 1.19508 8073 0.2248 310 820.03 607.53 1.05741 1083 1.060 5000 1392.9 1050.1 1.20129 8837 0.2096 3150 834.75 618.82 1.06212 1161 1.006 5100 1423.6 1074.0 1.20738 965 0.1956 3200 849.48 630.12 1.06676 1242 0.9546 5200 1454.4 1098.0 1.21336 10539 0.1828 3250 864.24 641.46 1.07134 1328 0.9069 5300 1485.3 1122.0 1.21923 11481 0.1710 3300 879.02 652.81 1.07585 1418 0.8621 3350 893.83 664.20 1.08031 1513 0.8202 3400 908.66 675.60 1.08470 1613 0.7807 3450 923.52 687.04 1.08904 1719 0.7436 3500 938.40 698.48 1.09332 1829 0.7087 3550 953.30 709.95 1.09755 1946 0.6759 3600 968.21 721.44 1.10172 2068 0.6449 3650 983.15 732.95 1.10584 2196 0.6157