Question
Hi, Im having trouble with this question. I worked it out but ended up getting a weird value. I made a table which gave me
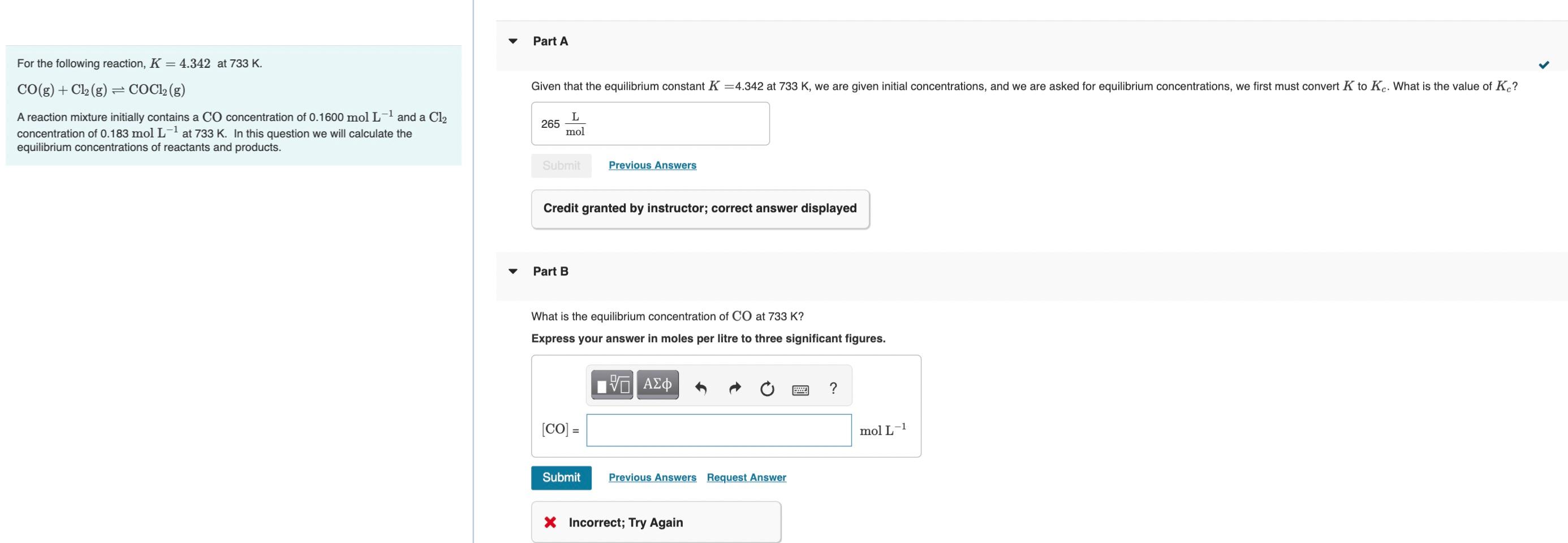 Hi, Im having trouble with this question. I worked it out but ended up getting a weird value. I made a table which gave me 0.1600-x and 0.183-x. I plugged everything into k = [COCl2]/[CO][Cl2] I rearranged and expanded ending up with 4.342x2 - 1.47x + 0.127 = 0. I then plugged in the values into -b +- squroot b2 - 4ac / 2a
Hi, Im having trouble with this question. I worked it out but ended up getting a weird value. I made a table which gave me 0.1600-x and 0.183-x. I plugged everything into k = [COCl2]/[CO][Cl2] I rearranged and expanded ending up with 4.342x2 - 1.47x + 0.127 = 0. I then plugged in the values into -b +- squroot b2 - 4ac / 2a
I got 1.47 +- squroot 2.16 - 2.20 , it cant be a negative number so I rounded to get 2.2 - 2.2 which would give me 0 in the end after plugging in the values I ended up with 0.169. This doesnt work because subtracting 0.169 from 0.1600 cant be a negative value. Any help on how to solve this would be appreciated
For the following reaction, K=4.342 at 733K. CO(g)+Cl2(g)COCl2(g) A reaction mixture initially contains a CO concentration of 0.1600molL1 and a Cl2 concentration of 0.183molL1 at 733K. In this question we will calculate the equilibrium concentrations of reactants and products. Credit granted by instructor; correct answer displayed Part B What is the equilibrium concentration of CO at 733K ? Express your answer in moles per litre to three significant figures. 3 Incorrect; Try AgainStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


