Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi. Please help me to find out the hypothetical path of non reactor (e.g Quench Tank). Don't spam. 3.0 DESCRIPTION The oxychlorination process design is
Hi. Please help me to find out the hypothetical path of non reactor (e.g Quench Tank). 
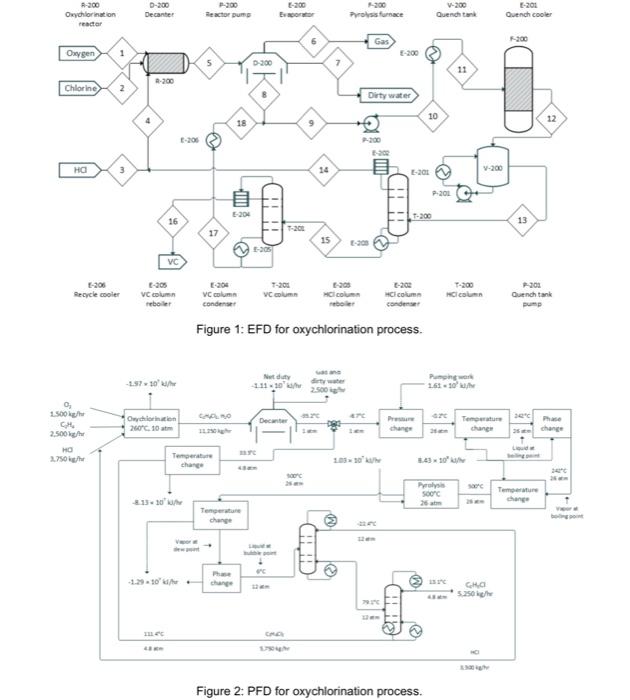
3.0 DESCRIPTION The oxychlorination process design is highly comparable to the direct chlorination method, with many shared downstream operations (Benyahia, 2005). It begins with preheated (from storage at 25C) near-stoichiometric feeds of pure ethylene, oxygen, and hydrogen chloride (streams 1,2, and 3; stream 3 joins with a recycle stream to form stream 4) entering an oxychlorination reactor (R-200). Oxychlorination reactors are gas phase reactors, which avoid corrosion by aqueous acid solutions (a classic pitfall of direct chlorination reactors) (Magistro & Cowfer, 1986). To maintain a vapor fraction of 1, the reactor is held at 260C and 10 atm. The oxychlorination products (stream 5) are flown to a decanter (D-200), which removes gas (stream 6) and water (stream 7). The bottoms stream from the decanter, stream 8, is joined by a recycle (to form stream 9) before entering a centrifugal pump and heating system in preparation for pyrolysis (F-200; streams 10 and 11). As with the direct chlorination method, the pyrolysis furnace runs at 500C and 26 atm before opening into a quench tank (V-200; stream 12) at 12 bar and 6C. The quench tank temperature is maintained by continuous material circulation in contact with a refrigerant stream. After cooling, the products travel to distillation columns that are essentially identical to those in the direct chlorination process (T-200 and T-201; streams 13 and 15), which one major deviation. In the oxychlorination model, the distillate HCl from T-200 (stream 14) is recycled upstream to enter the oxychlorination reactor alongside the pure components. Similar to the direct chlorination process, the distillate from T-201 (stream 16) is 98% pure vinyl chloride at 13C and 4.8 atm while the bottoms (stream 17), which is primarily water and 1,2-dichloroethane, is recycled to merge with the enriched 1,2-dichloroethane exiting the bottom of the decanter. The EFD and PFD for oxychlorination process is given in Figure 1 and 2, respectively. Summaries for all the process is given in Table 1. E-200 D-200 Decanter Reactorum -300 Profurnace V-200 Quench Oxychlorination reactor E-201 Quench cooler Gas Owygen 1 -200 D300 -200 Chlorine 2 Dirty water 10 12 18 01 3 14 V-200 L-201 P-200 E-204 T-200 16 13 17 15 VC -30 -205 Recycle cooler -205 VC cum ber VC column condenser Column CI -202 colum condenser T-200 Cicon 201 Quench tank pump Figure 1: EFD for oxychlorination process. Nud -2.5710 Pung 161-10 Owych 2007.10 Decanter O 1.500 CH 2.500 kg/h 2.750 change change change Loud Temperature 2010'UN 3.430 SOC Teme change Temperature change VE change CHO 5.250 HO Figure 2: PFD for oxychlorination process. 3.0 DESCRIPTION The oxychlorination process design is highly comparable to the direct chlorination method, with many shared downstream operations (Benyahia, 2005). It begins with preheated (from storage at 25C) near-stoichiometric feeds of pure ethylene, oxygen, and hydrogen chloride (streams 1,2, and 3; stream 3 joins with a recycle stream to form stream 4) entering an oxychlorination reactor (R-200). Oxychlorination reactors are gas phase reactors, which avoid corrosion by aqueous acid solutions (a classic pitfall of direct chlorination reactors) (Magistro & Cowfer, 1986). To maintain a vapor fraction of 1, the reactor is held at 260C and 10 atm. The oxychlorination products (stream 5) are flown to a decanter (D-200), which removes gas (stream 6) and water (stream 7). The bottoms stream from the decanter, stream 8, is joined by a recycle (to form stream 9) before entering a centrifugal pump and heating system in preparation for pyrolysis (F-200; streams 10 and 11). As with the direct chlorination method, the pyrolysis furnace runs at 500C and 26 atm before opening into a quench tank (V-200; stream 12) at 12 bar and 6C. The quench tank temperature is maintained by continuous material circulation in contact with a refrigerant stream. After cooling, the products travel to distillation columns that are essentially identical to those in the direct chlorination process (T-200 and T-201; streams 13 and 15), which one major deviation. In the oxychlorination model, the distillate HCl from T-200 (stream 14) is recycled upstream to enter the oxychlorination reactor alongside the pure components. Similar to the direct chlorination process, the distillate from T-201 (stream 16) is 98% pure vinyl chloride at 13C and 4.8 atm while the bottoms (stream 17), which is primarily water and 1,2-dichloroethane, is recycled to merge with the enriched 1,2-dichloroethane exiting the bottom of the decanter. The EFD and PFD for oxychlorination process is given in Figure 1 and 2, respectively. Summaries for all the process is given in Table 1. E-200 D-200 Decanter Reactorum -300 Profurnace V-200 Quench Oxychlorination reactor E-201 Quench cooler Gas Owygen 1 -200 D300 -200 Chlorine 2 Dirty water 10 12 18 01 3 14 V-200 L-201 P-200 E-204 T-200 16 13 17 15 VC -30 -205 Recycle cooler -205 VC cum ber VC column condenser Column CI -202 colum condenser T-200 Cicon 201 Quench tank pump Figure 1: EFD for oxychlorination process. Nud -2.5710 Pung 161-10 Owych 2007.10 Decanter O 1.500 CH 2.500 kg/h 2.750 change change change Loud Temperature 2010'UN 3.430 SOC Teme change Temperature change VE change CHO 5.250 HO Figure 2: PFD for oxychlorination process Don't spam.


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


