Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hi, please show what equations are used to calculate. i will rate! here are the updated photos, thanks! (2pts) Enthalpy and Entropy Changes of Dissolving
hi, please show what equations are used to calculate. i will rate! 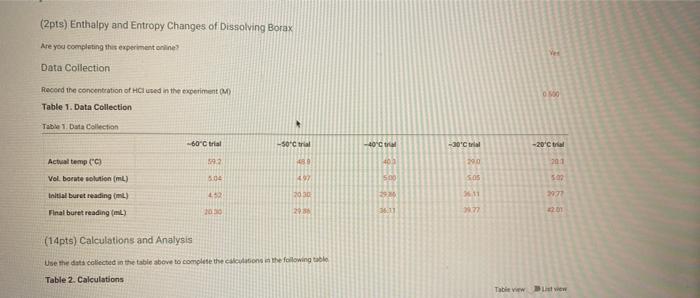




here are the updated photos, thanks! 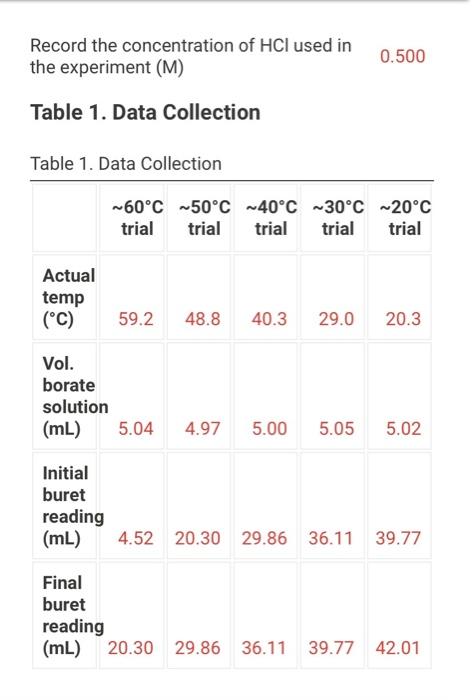
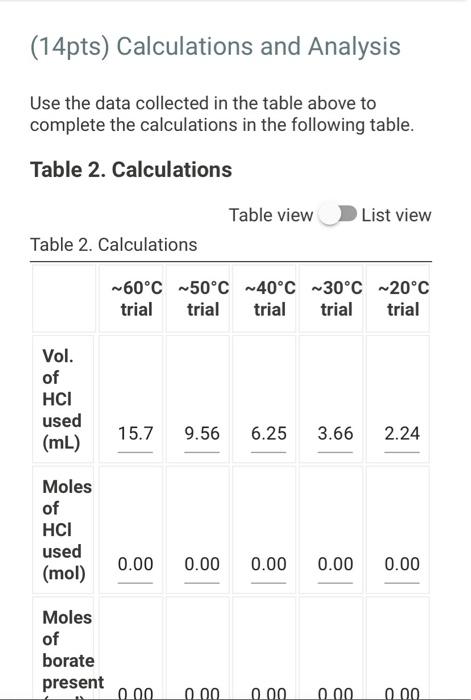
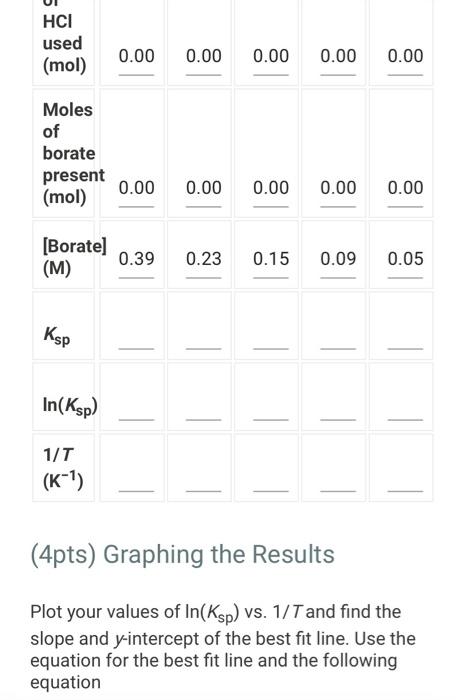

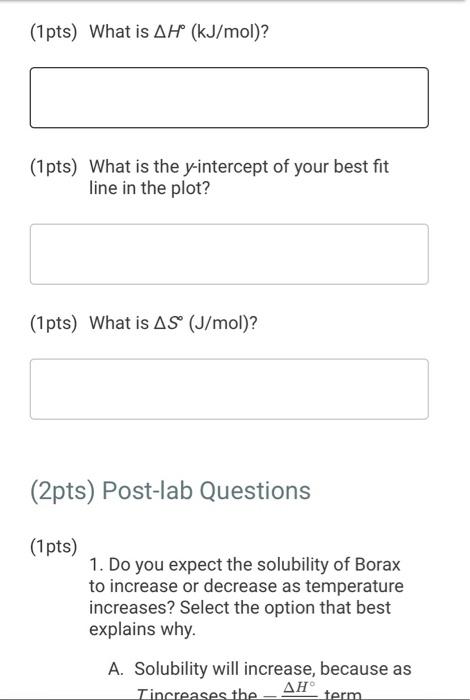


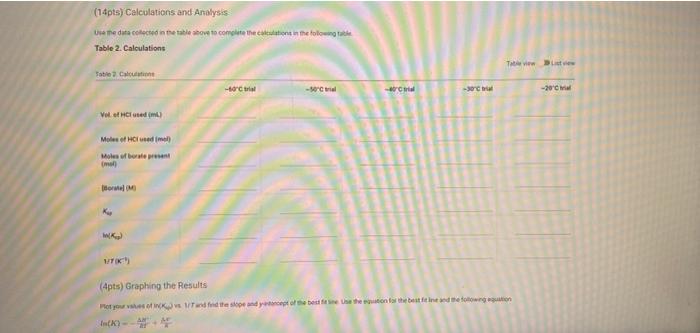
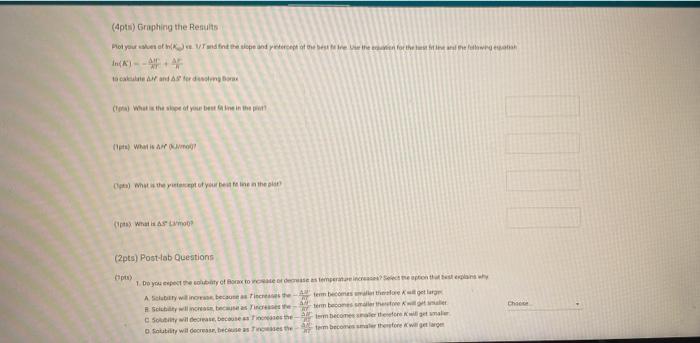
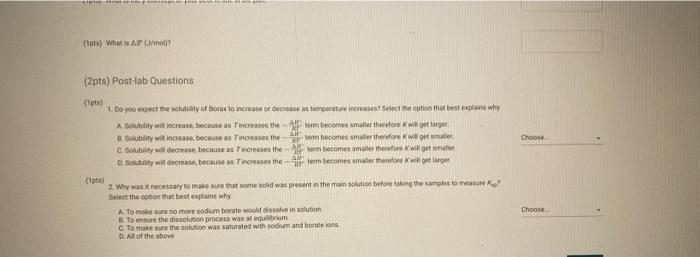
(2pts) Enthalpy and Entropy Changes of Dissolving Borax Are you completing this experimentalne Ves Data Collection Record the concentration of HClused in the experiment Table 1. Data Collection Table 1. Data Collection -60C trial -SOC trial 40' -30'C tri -20 cm 2 080 403 200 201 Actualtemp (c) Volborte solution (m) Initial buret reading (m) 5.04 500 505 442 2030 29 351 71 100 Final buret reading (m) 293 30.11 977 2.1 (14pts) Calculations and Analysis Use the data collected in the table stove to complete the actions in the following table Table 2. Calculations Tablew w (14pts) Calculations and Analysis Use the data collected in the table store to complete the cautions in the following the Table 2. Calculations Fabie Calculation views -trial - 50 - - -200 Vetemed (L) Moleto de Mole of bone WYK (Apts) Graphing the Results For you and find the sound of the best the the best in the following (X) - (Apts) Graphing the Results Prol your head to the slope and treat weekend for the best in two In) -AP to care and After one ( What the shoot your best tine in the ) WATT C) whethemetot you in the cm wall apo (2pts) Post-lab Questions Chce 1. Do you the best of orato vendedores en este A Solubility will now because the rem becomes with all get from Seby water becomes Sy wil dereceases the am become there Soutility wilderness them becomes therefore we get (pt) What Asmo (2pts) Post-lab Questions ots) 1. Do you expect the solubaty of Born to increase orders temperature increase Select the option that best explains why A Solubility will increase because an increases the Allem becomes maller therefore will get langer B Solubility will increase because as increases the AR Sem become smaller therefore will get a Solubility will because as Tresses the term becomes maller therefore will get a Solubility wil decreas, becauses increases the term becomes mer herefore will Choose 2. Why was necessary to make sure that comes was present in the main solution before taking the samples to mark? Select the option that beat explains why A To make sure no more sodium borate would dissolve in solution B. To ensure the dissolution process was at equilibrium To make sure the solution was saturated with sodium and bones Al of the above Choone Record the concentration of HCI used in the experiment (M) 0.500 Table 1. Data Collection Table 1. Data Collection ~60C~50C~40C ~30C ~20C trial trial trial trial trial Actual temp (C) 59.2 48.8 40.3 29.0 20.3 Vol. borate solution (mL) 5.04 4.97 5.00 5.05 5.02 Initial buret reading (mL) 4.52 20.30 29.86 36.11 39.77 Final buret reading (mL) 20.30 29.86 36.11 39.77 42.01 (14pts) Calculations and Analysis Use the data collected in the table above to complete the calculations in the following table. Table 2. Calculations Table view List view Table 2. Calculations ~60C~50C~40C ~30C 20C trial trial trial trial trial Vol. of HCI used 15.7 9.56 6.25 3.66 2.24 (mL) Moles of HCI used 0.00 0.00 0.00 0.00 0.00 (mol) Moles of borate present 0.00 0.00 0.00 0.00 0.00 HCI used (mol) 0.00 0.00 0.00 0.00 0.00 Moles of borate present (mol) 0.00 0.00 0.00 0.00 0.00 [Borate! (M) M 0.39 0.23 0.15 0.09 0.05 sp In(Ksp) 1/T (K-1) (4pts) Graphing the Results Plot your values of In(Ksp) vs. 1/T and find the slope and y-intercept of the best fit line. Use the equation for the best fit line and the following equation (4pts) Graphing the Results Plot your values of In(Ksp) vs. 1/T and find the slope and y-intercept of the best fit line. Use the equation for the best fit line and the following equation In(K) = -1 AH AS + R to calculate AH and AS for dissolving Borax (1pts) What is the slope of your best fit line in the plot? (1 pts) What is AH (kJ/mol)? (1 pts) What is the y-intercept of your best fit line in the plot? (1 pts) What is AH (kJ/mol)? (1pts) What is the y-intercept of your best fit line in the plot? (1 pts) What is AS (J/mol)? (2pts) Post-lab Questions (1 pts) 1. Do you expect the solubility of Borax to increase or decrease as temperature increases? Select the option that best explains why. A. Solubility will increase, because as Tincreases the AH term (2pts) Post-lab Questions (1pts) 1. Do you expect the solubility of Borax to increase or decrease as temperature increases? Select the option that best explains why. A. Solubility will increase, because as Tincreases the AH term RT becomes smaller therefore K will get larger. B. Solubility will increase, because as AHO Tincreases the term RT becomes smaller therefore K will get smaller. C. Solubility will decrease, because AH as Tincreases the term RT becomes smaller therefore K will get smaller. D. Solubility will decrease, because AH as Tincreases the term RT becomes smaller therefore K will get larger. Choose... (1 pts) 2. Why was it necessary to make sure that some solid was present in the main solution before taking the samples to measure Ksp? Select the option that best explains why. A. To make sure no more sodium borate would dissolve in solution. B. To ensure the dissolution process was at equilibrium. C. To make sure the solution was saturated with sodium and borate ions. D. All of the above Choose... Total:-/22 pts You have 2 attempts remaining. SUBMIT 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


