Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi there i am having extreame difficulty doing pre lab homework can i please get help on the questions i have circled down below. please
Hi there i am having extreame difficulty doing pre lab homework can i please get help on the questions i have circled down below.
please solve the questions providing detailed solution so i can take notes and understand and better prepare for an actual lab
note-please provide details as you solve the problems so i can take notes too and learn please solve the questions curcled in red. Its only one red circle
**please solve the following problem so i can learn and understand from your answer
**I put a red curcle on the calculations question i want to learn .Solve them in step by step so i can learn from you.
**I have provided three imajes of the same worksheet but with better and clear imajes to help you .
**If information is unclear let me know .
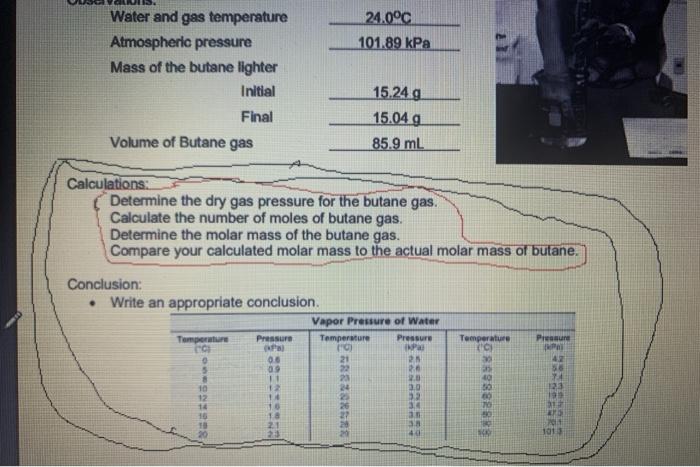
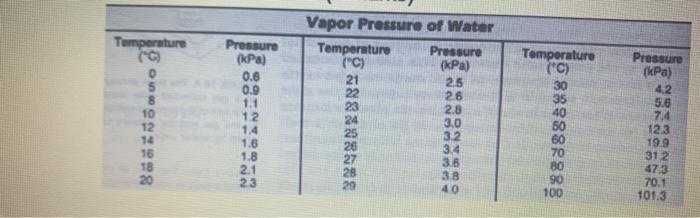
*Determine the dry gas pressure for the butane gas.
*calculate the number of moles of butane gas .
*Determine the molar mass of the Butane gas.
*Compare your calculated molar mass to the actual molar mass of butane.
*Lastly just show me how i can write a nice conclusion on this lab .
thanks so much and ill leave a thumbs up :)

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


