Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi there, I would really appreciate full worked soultioms to all parts of question 4. I have also included a photo of the answers. Thank
Hi there, I would really appreciate full worked soultioms to all parts of question 4.
4. (a) An air compressor takes air at ambient conditions (20C and 1 bar ) and inflates an initially flat tyre (pressure =1 bar) to a pressure of 3.2 bar. If the process is assumed to be isentropic, calculate the temperature of the air in the tyre. Sketch the process on a T-S diagram. [4 marks] (b) In practice the process in part (a) is not isentropic and the air temperature is measured to be 145C after inflation to 3.2 bar. Calculate the isentropic efficiency of the process. Sketch the process on a T-S diagram. [4 marks] (c) If the volume of the tyre is 15 litres, calculate how much extra mass of air enters the tyre in the isentropic case in part (a) compared to the actual [4 marks] case in part (b). (d) Calculate the entropy change of the air used to inflate the tyre. [5 marks] (e) Following a period of time, the air inside the tyre cools down to ambient temperature. What is the final pressure in the tyre? [2 marks] (f) If the desired final pressure in the tyre was 3.2 bar, what pressure should the tyre be pumped up to before the air cools down, assuming the compression process was isentropic? [6 marks] 4 a) 409K, b) 0.924, c) 0.9g, d) 0.92J/K, e) 2.24bar, f) 5.1 bar I have also included a photo of the answers. 
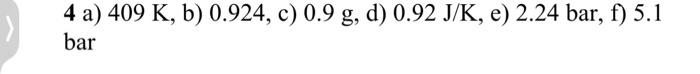
Thank you so much

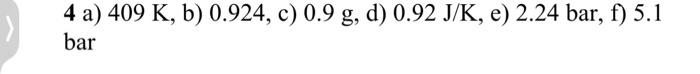
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


