Question: Hi this is my THIRD time uploading this, the last time, the tutor did not even take the time to actually do it and Took




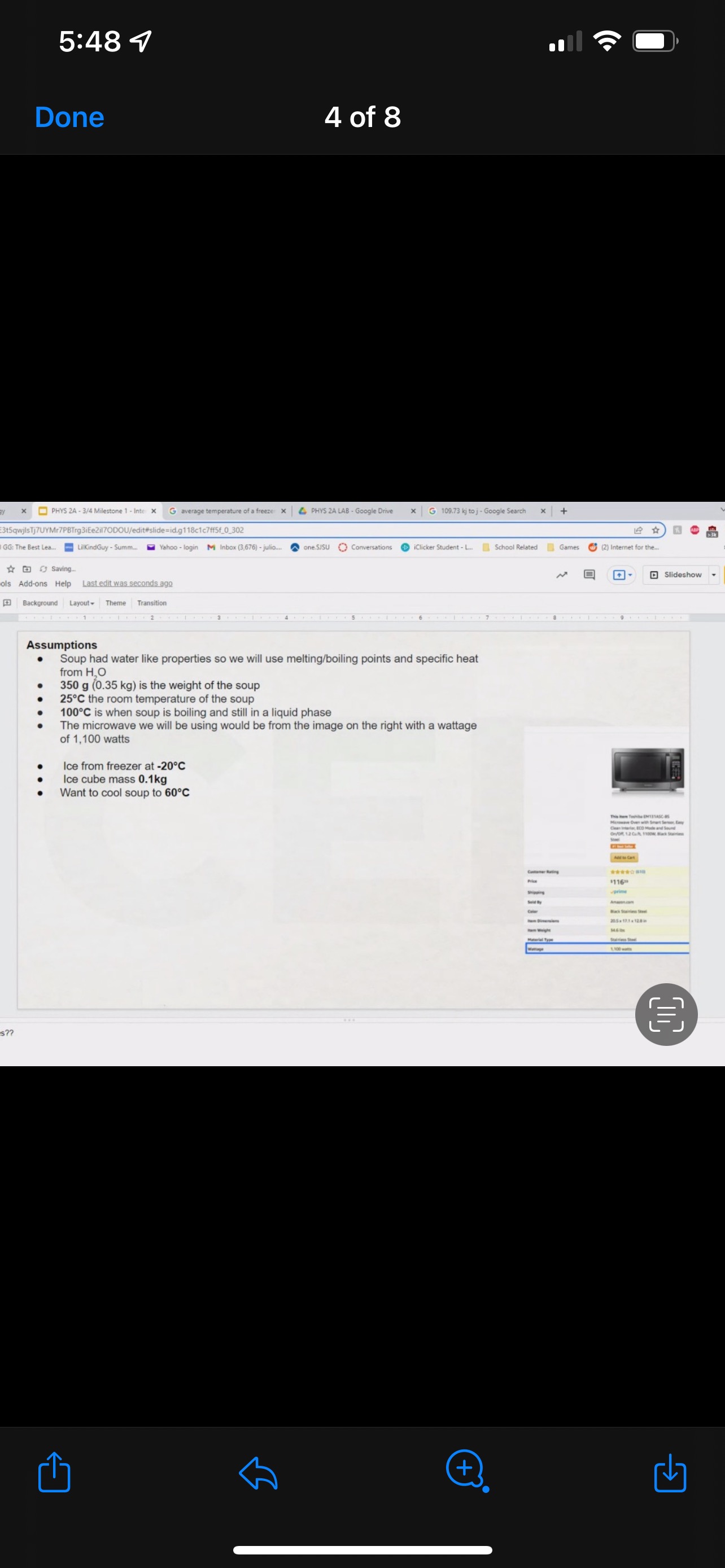
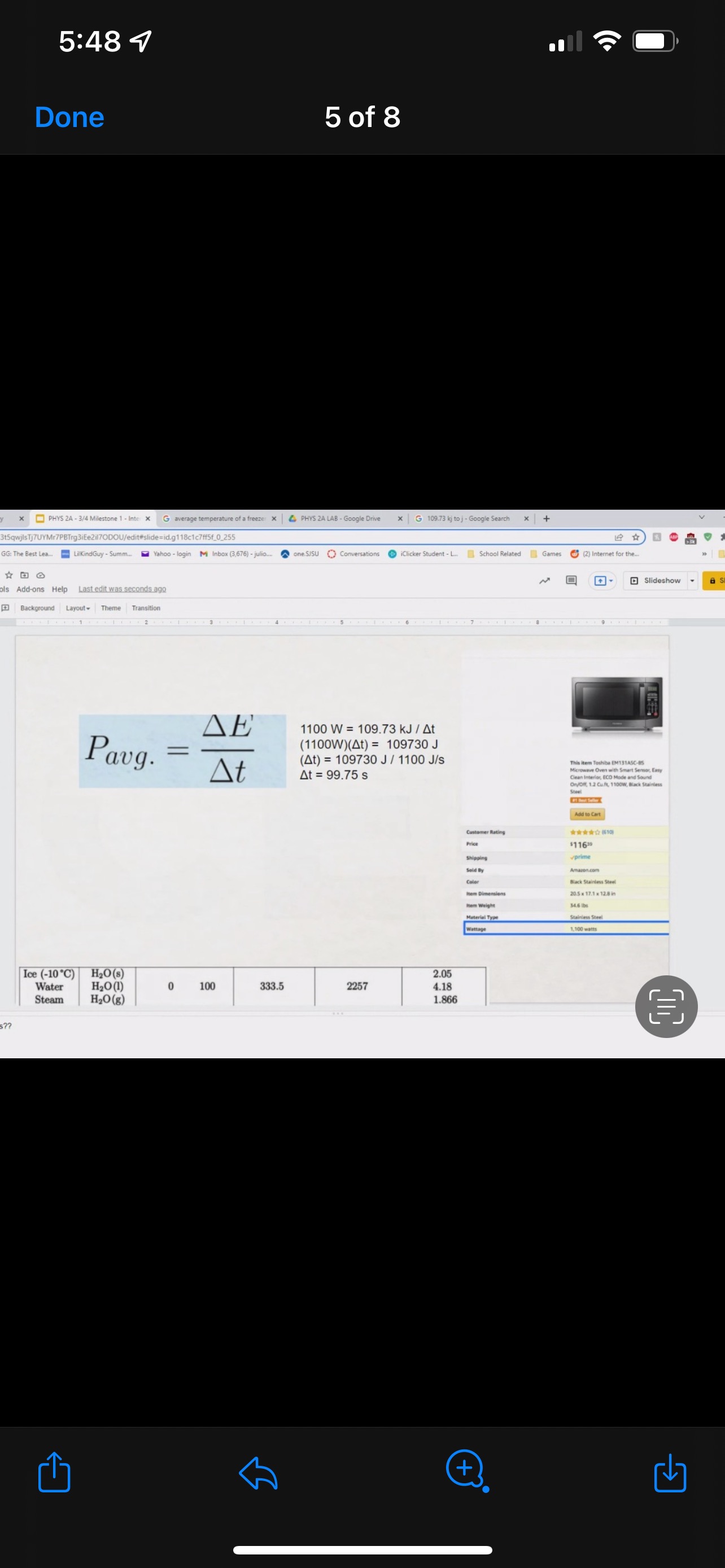
Hi this is my THIRD time uploading this, the last time, the tutor did not even take the time to actually do it and Took almost 4 hours since everyone seem to not like the long questions. The first 3 image is the prompt and the question. the last 5 picture is an example of how it can be. the question does not provide all the numbers so we need to just make an assumption based on the image they show and just by looking it up on google. it does not need to be a specific one but just a number that you can look up like for example look up a micrwave watt and detail of the soup or even just any make up assumption number. Not even sure if the example pics I provided are correct but it will all just be your assumption number but the lay out is what I am to show. we need to also add a little story like begining and middle and end if possible and also add the circle diagram and the graphs. it does not need to be long.
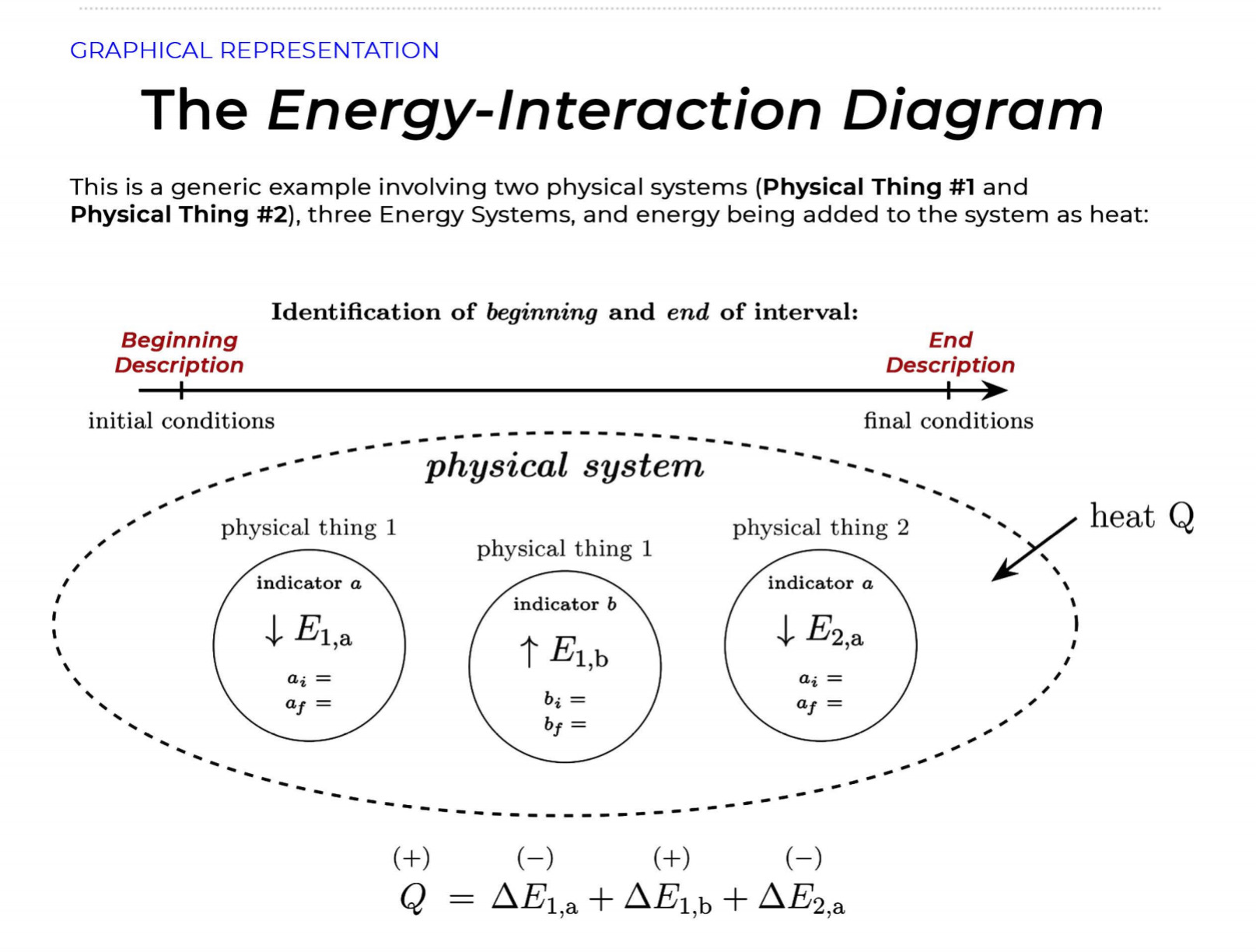
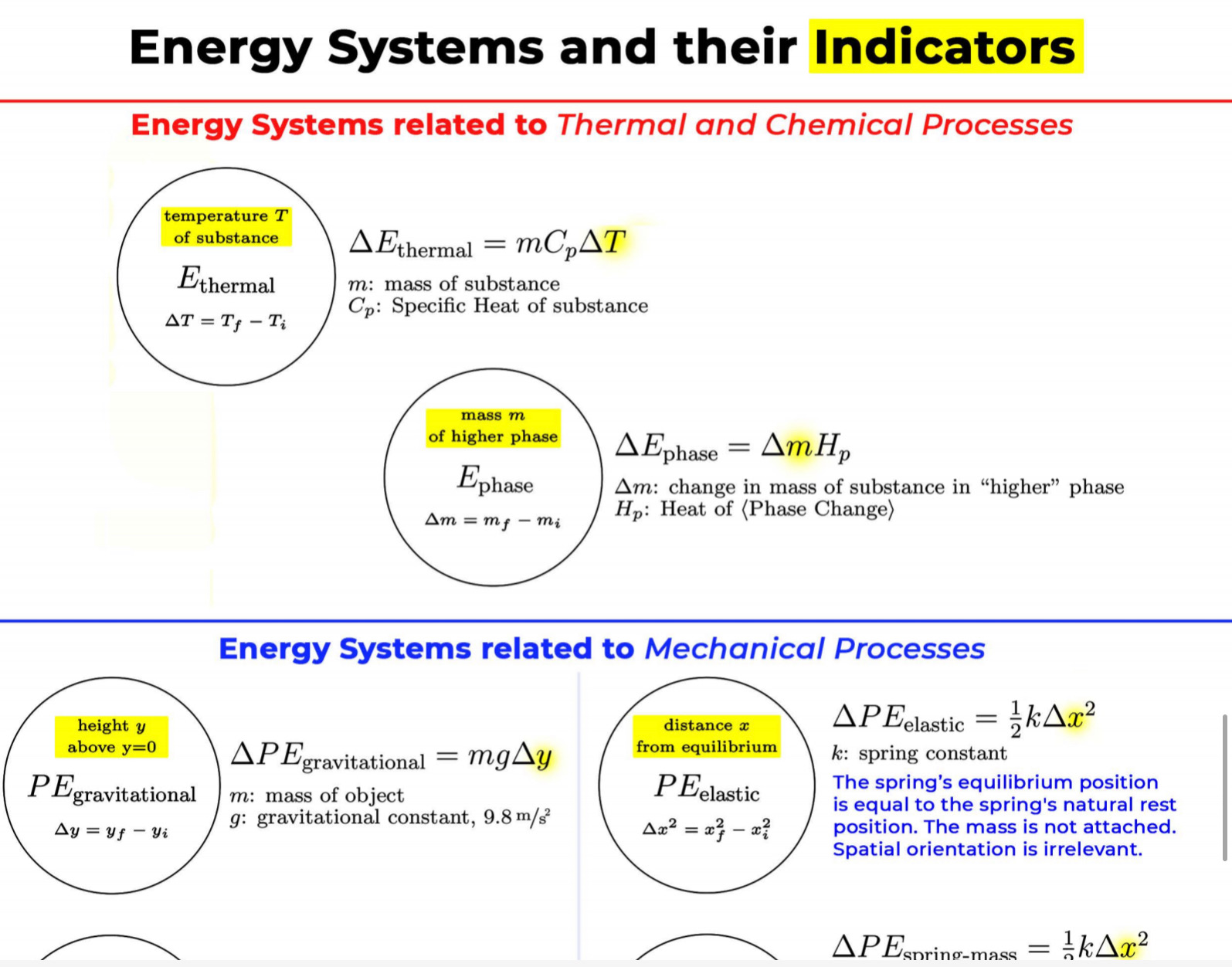




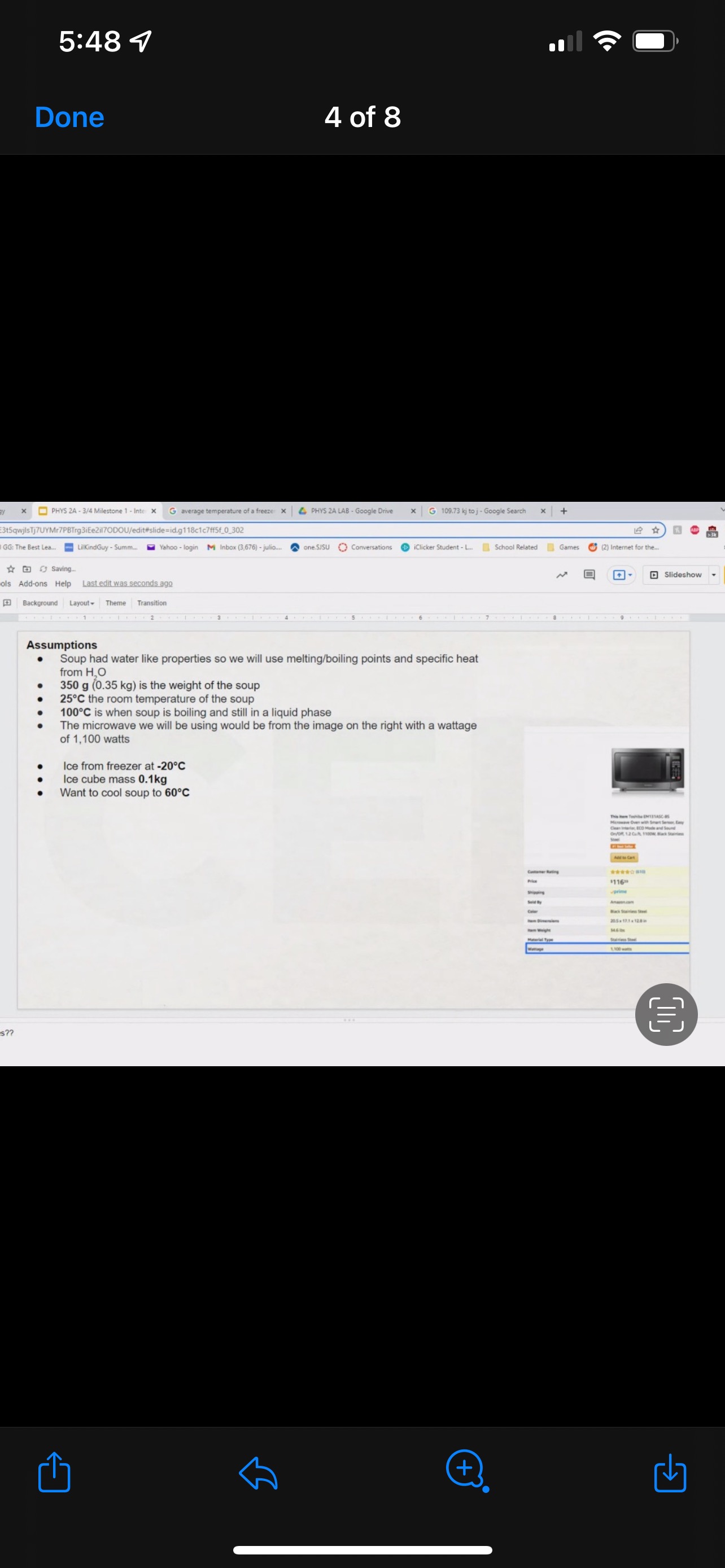
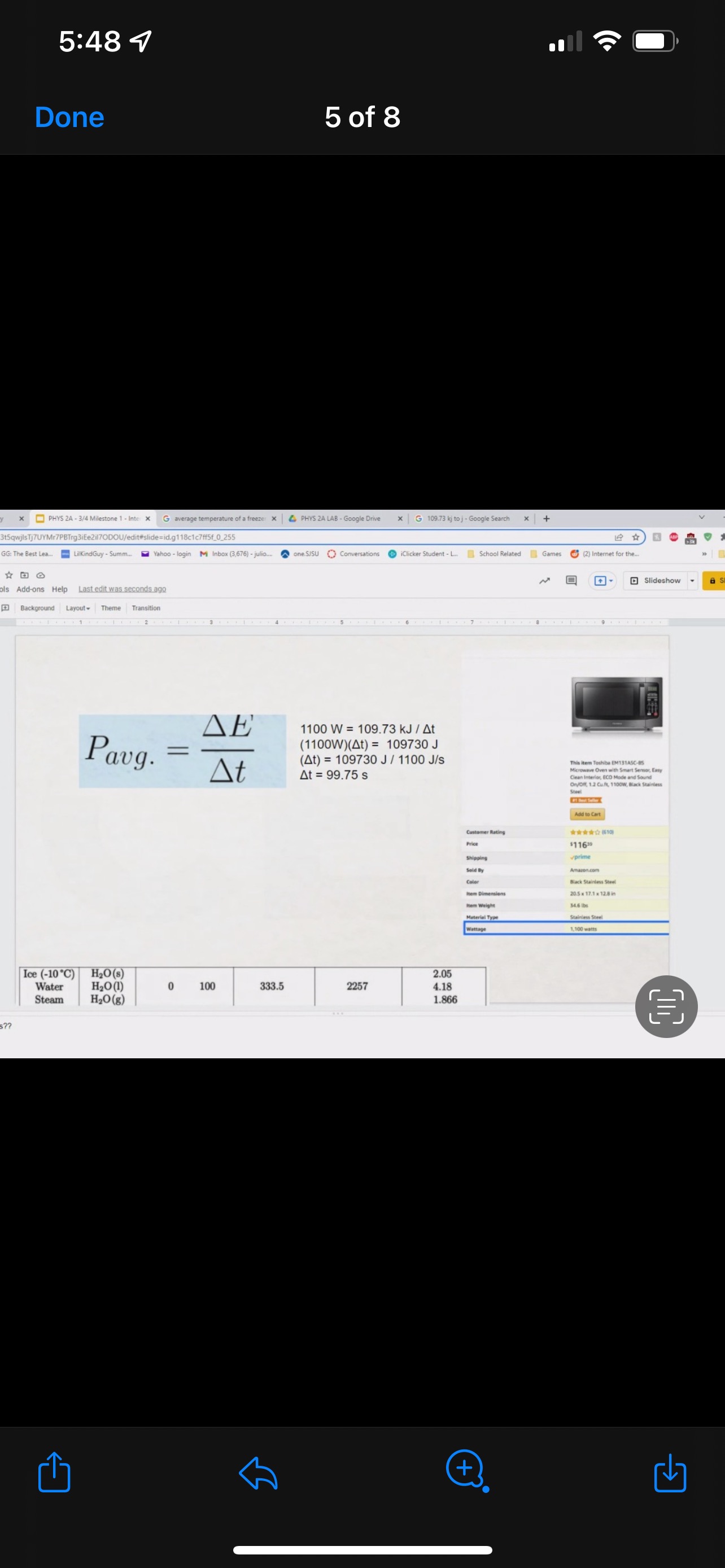
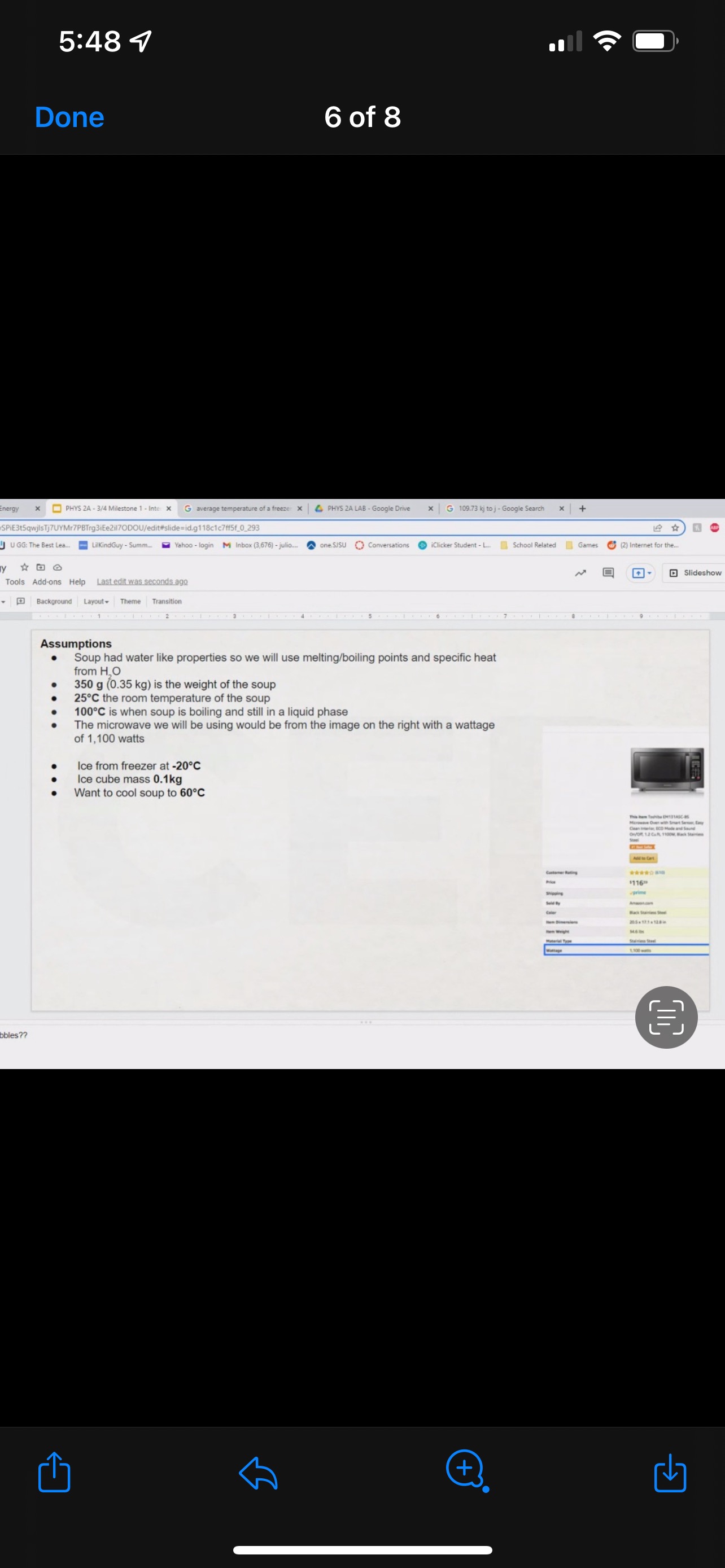
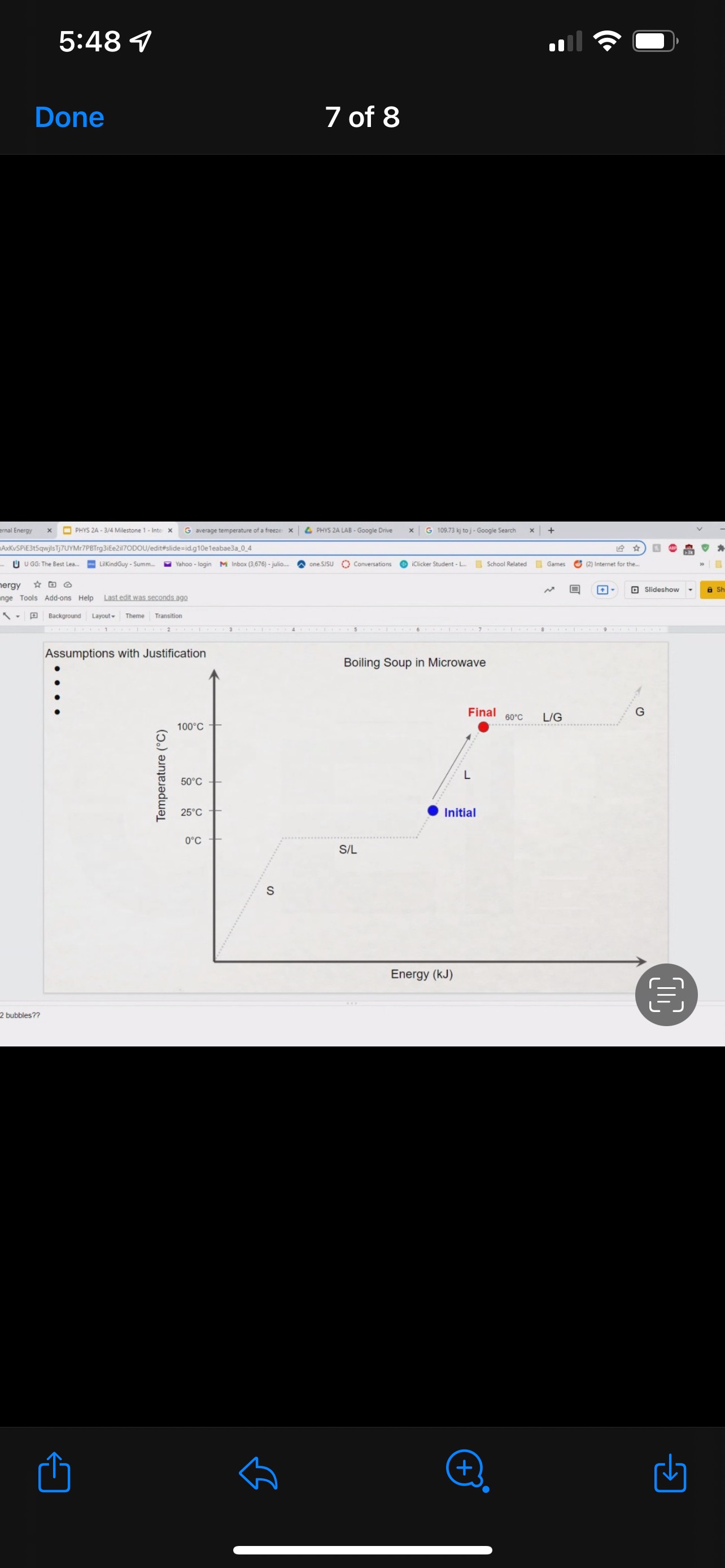
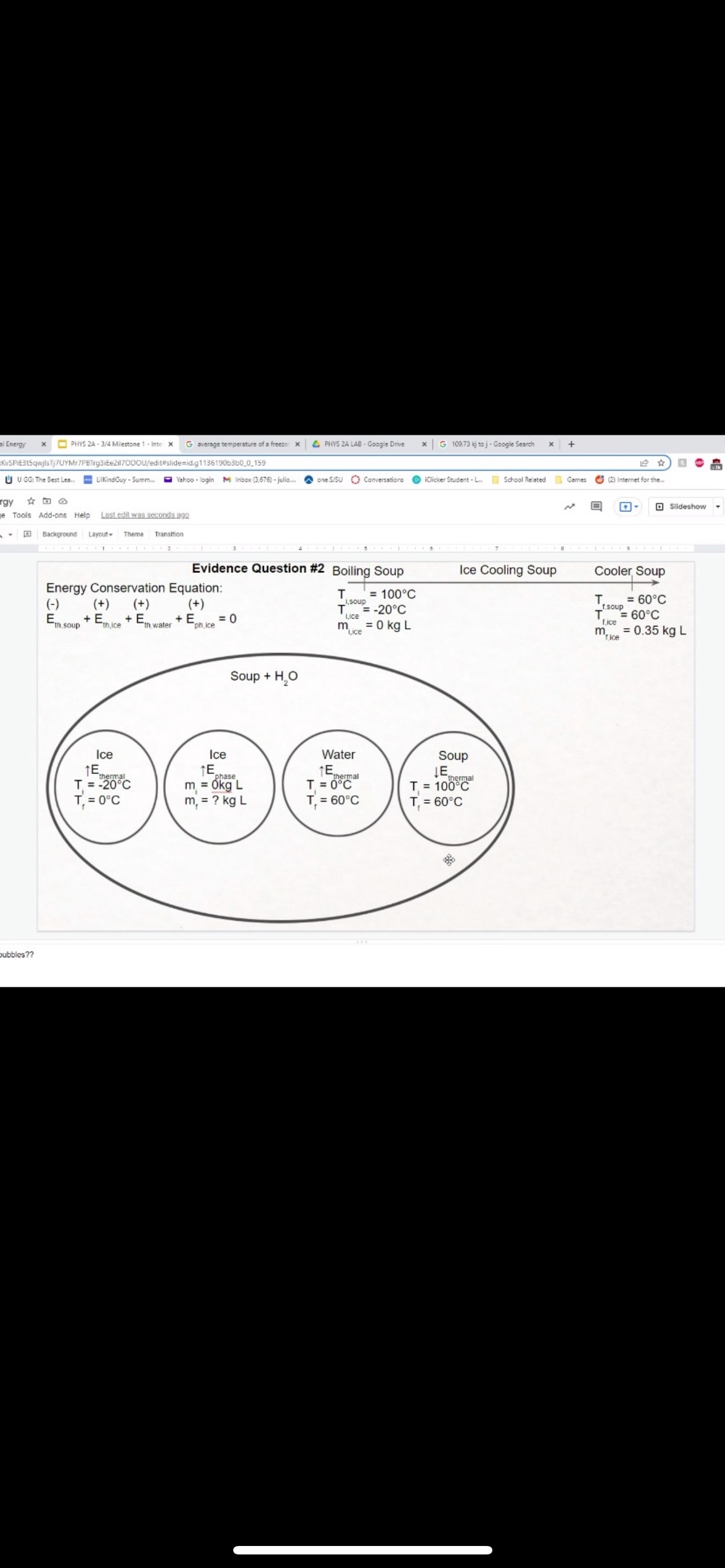
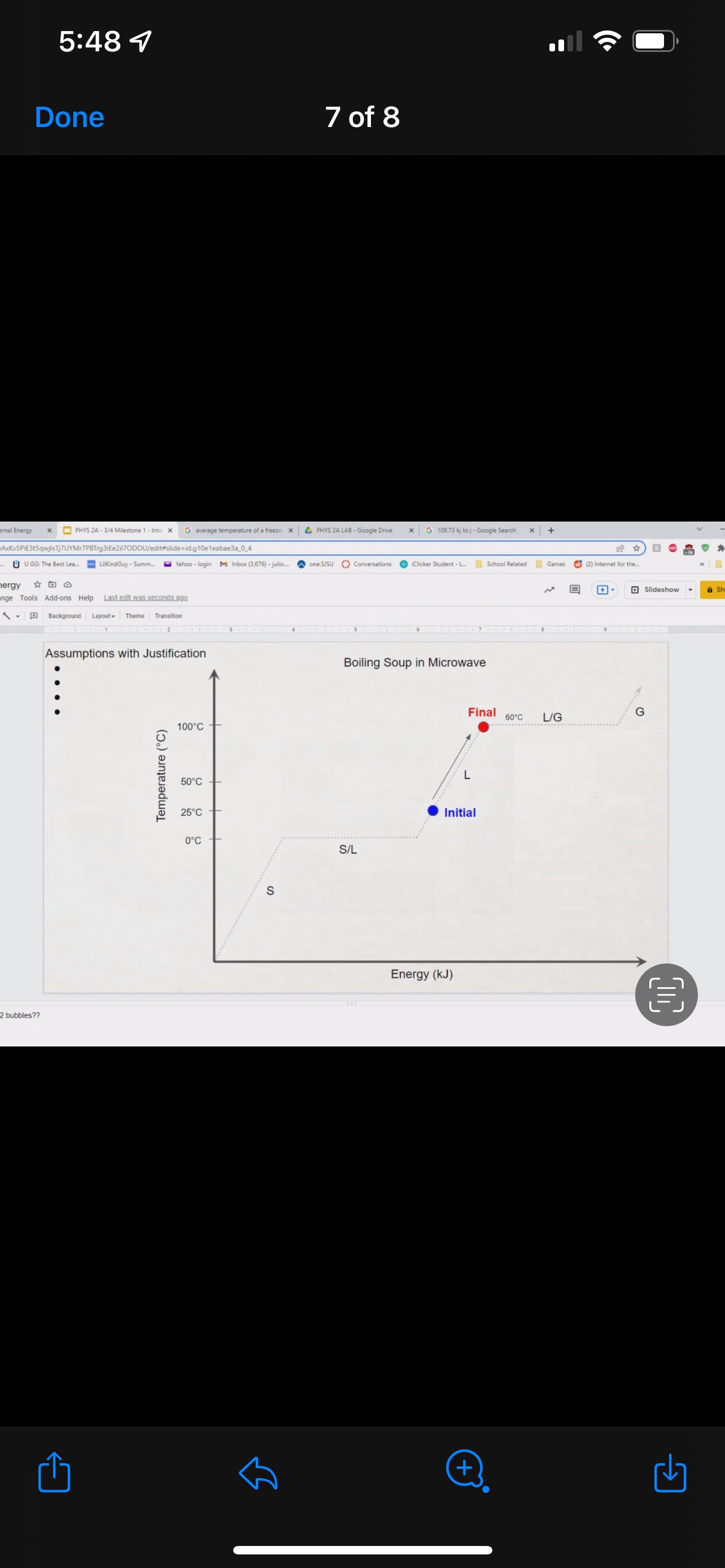
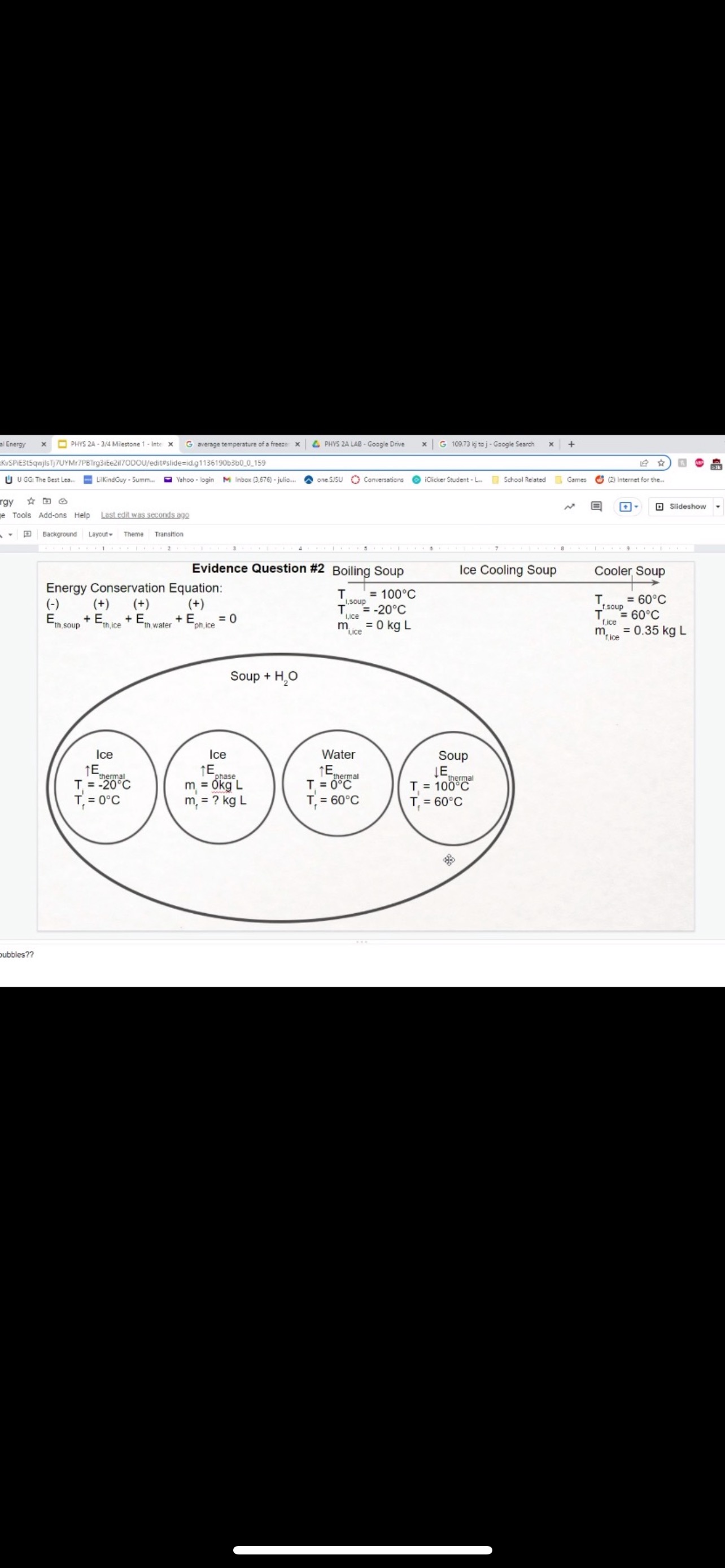
GRAPHICAL REPRESENTATION The Energy-Interaction Diagram This is a generic example involving two physical systems (Physical Thing #1 and Physical Thing #2), three Energy Systems, and energy being added to the system as heat: Identification of beginning and end of interval: Beginning End Description Description initial conditions final conditions physical system physical thing 1 physical thing 2 heat Q physical thing 1 indicator a indicator a indicator b I El,a 1 El,b I E2,a ai Or = of = bi = of bf = ( + ) (- ) ( + ) (- ) Q = AEl,a + AEl,b + AE2,aEnergy Systems and their Indicators Energy Systems related to Thermal and Chemical Processes temperature T of substance A Ethermal = mCpAT Ethermal m: mass of substance AT = TJ - Ti Cp: Specific Heat of substance mass m of higher phase A Ephase = AmHp Ephase Am: change in mass of substance in "higher" phase Am = my - mi Hp: Heat of (Phase Change) Energy Systems related to Mechanical Processes height y distance x AP Eelastic = kAx2 above y=0 AP Egravitational = mgy from equilibrium k: spring constant PEgravitational m: mass of object PEelastic The spring's equilibrium position is equal to the spring's natural rest Ay = yf - yi g: gravitational constant, 9.8 m/s Ax2 = 27 -27 position. The mass is not attached. Spatial orientation is irrelevant. AP Espring-mass = KAx2MILESTONE #1 INTERNAL ENERGY You prepare some Campbell's tomato soup by mixing the contents of one can of soup with one can of water. Then, using your favorite insulated cup, you heat the soup in your microwave until the soup is just boiling. This allows the fats in the soup to melt and ensures that your soup is delicious. From past experience, you know that the soup is too hot to have immediately without burning your mouth. But because you're impatient, you want to add ice (taken directly from your freezer) to your soup so that it quickly cools to a safe and enjoyable temperature. The Big Question is: How should you prepare your soup so that it's delicious and enjoyable? Specifically, (1) how long should you microwave your soup so that it just starts boiling, and (2) how many ice cubes should you add to reach the enjoyable temperature? In case you need some resources, here's a link to our P_hysics Formalisms. Your task is to answer the Big Question in the form of an argument using the Claim, Evidence, Reasoning framework. To do this, you will need to model cooling the soup in order to come up with a quantitative prediction of how many ice cubes you should add. To do this, you will need to model cooling the soup in order to come up with a quantitative prediction of how many ice cubes you should add. Be sure to include any relevant: Assumptions with Justifications: These should be clearly stated (ie in a PURPLE text box) near the beginning of your solution. Justification Annotations: These should be included using text boxes throughout your solution explaining why you made the particular choices that you did for your representations AND describe how the representations are consistent with one another. Graphical Representations: These must be complete (ie. have titles with units, have all relevant components, etc.) as shown on the model sheets in Modules. Scroll down from the "Course Information" heading until you see "Model Sheets"). Algebraic Representations: These should have terms with relevant subscripts and appear in an easy to follow, logical manner that culminate in a quantitative prediction used to answer the Big Question. Be sure to include any additional definitions from physics formalisms that you use and explicitly show any physical quantity conversions. Argument(s): Specifically, the answer to the Big Question must be in the form of an Argument using the CER framework. Below are some questions and prompts to help in case you get stuck. Guiding Questions and Prompts: You do not need to answer these questions explicitly (i.e. provide a list of answers). However, your overall solution should demonstrate that you know all of these answers. 1. 2. . What additional information about tomato SOLID and ice do you need in order to have a complete Tell a story of cooling the tomato soup. What information do you know at the beginning and end of the story? What representation will help you tell a story? How many of these representations might you need? representation of each substance? Is there a way you can answer this question by making assumptions? How might you justify each of these? . Tomato soup consists of tomato soup concentrate and water. How might you use this information to model tomato soup? Ice is made of water that comes from the freezer. How might you model the ice cooling the soup? . What representation(s) can help you construct an algebraic representation that will allow you to come up with a quantitative prediction about the number of ice cubes you need? . What does your final quantitative answer mean? How will you answer the Big Question based on your Evidence? What Reasoning will you include? To do this, you will need to model cooling the soup in order to come up with a quantitative 30f7 prediction of how many ice cubes you should add. Be sure to include any relevant: o Assumptions with Justifications: These should be clearly stated (is in a PURPLE text box) near the beginning of your solution. 0 Justification Annotations: These should be included using text boxes throughout your solution explaining why you made the particular choices that you did for your representations AND describe how the representations are consistent with one another Graphical Representations: These must be complete (i.e. have titles with units, have all relevant components, etc.) as shown on the model sheets in MM;- Scroll down from the "Course Information" heading until you see "Model Sheets"). Algebraic Representations: These should have terms with relevant subscripts and appear in an easy to follow, logical manner that culminate in a quantitative prediction used to answer the Big Question. Be sure to include any additional definitions from physics formalisms that you use and explicitly show any physical quantity conversions. o Argument(s): Specifically, the answer to the Big Question must be in the form of an Argument using the CER framework. 5:48 4 Done 4 of 8 * PHYS 2A - 3/4 Milestone 1 - Inte x G average temperature of a freeze: x 4 PHYS 2A LAB . Google Drive | G 109.73 kj to j - Google Search x |+ 315qwjisTj7UYMr7PBTrg3iEe2il70DOU/edit#slide=id.g118c1c7ff5f_0_302 GG: The Best Lea.. LilkindGuy - Summ.. Yahoo - login M Inbox (3,676) - julio.. one SISU () Conversations iclicker Student - L.. School Related Games (2) Internet for the. * @ Saving. Q Slideshow Add-ons Help Last edit was seconds ago Background Layout Theme Transition Assumptions Soup had water like properties so we will use melting/boiling points and specific heat rom HO 350 g (0.35 kg) is the weight of the soup 25C the room temperature of the soup 100 C is when soup is boiling and still in a liquid phase The microwave we will be using would be from the image on the right with a wattage of 1,100 watts ce from freezer at -20 C Ice cube mass 0.1kg Want to cool soup to 60 C prime + 35:48 4 Done 5 of 8 x ]PHYS 2A - 3/4 Milestone 1 - Inte x G average temperature of a freeze: x | PHYS 2A LAB - Google Drive x | G 109.73 kj to j - Google Search x|+ t5qwjlsTj7UYMr7PBTrg3ite2il70DOU/edit#slide=id.g118c1c7ff5f_0_255 GG: The Best Lea... LilkindGuy - Summ.. Yahoo - login M Inbox (3,676) - julio.. ~ one. SISU ()Conversations iClicker Student - L. School Related Games (2) Internet for the_. Q. Slideshow Is Add-ons Help Last edit was seconds ago Background Layout- Theme Transition AE 1100 W = 109.73 KJ / At Pavg. (1100W)(At) = 109730 J At (At) = 109730 J / 1100 J/s Toshiba EM131ASC At = 99.75 s Microwave Oven with Smart Sensor, Easy Clean Interior, ECO Mode and Sound OrOff 1.2 Cuft. 1 10ow, Black Stainless Add to Cart Customer Rating $11639 prime 20.5 x 17.1 x 12.8 in 34.6 1bs Stainless Steel 1.100 watts Ice (-10.C) H2O(s) 2.05 Water H2O (1) 100 333.5 2257 4.18 Steam H20(g) 1.866 + 35:48 4 Done 6 of 8 Energy PHYS 2A - 3/4 Milestone 1 - Inte x G average temperature of a freeze: * 4 PHYS 2A LAB - Google Drive * G 109.73 kj to j - Google Search 1+ SPIEStSqwjlsTj7UYMr7PBTrg3iEe2il70DOU/edit#slide=id.g118clc7ff5f_0_293 J U GG: The Best Lea. LilKindGuy - Summ... Yahoo - login M Inbox (3,676) - julio... one.SISU ()Conversations iClicker Student - L. School Related Games (2) Internet for the.. @ Slideshow Tools Add-ons Help Last edit was seconds ago Background Layout Theme Transition Assumptions Soup had water like properties so we will use melting/boiling points and specific heat from H, O 350 g (0.35 kg) is the weight of the soup 25' the room temperature of the soup 100 C is when soup is boiling and still in a liquid phase The microwave we will be using would be from the image on the right with a wattage of 1,100 watts Ice from freezer at -20 C Ice cube mass 0.1kg Want to cool soup to 60 C 116 prime 205. 1216 128mm bbles?? + 35:48 4 Done 7 of 8 mal Energy * PHYS 24 - 3/4 Milestone 1 - Inte x G average temperature of a freezer x 4 PHYS 2A LAB - Google Drive x G 109.73 kj to j - Google Search * + AxKvSPiE3t5qwjlsTj7UYMr7PBTrg3iEe2i170DOU/edit#slide=id.g10e leabae3a_0_4 U GG: The Best Lea.. LilKindGuy - Summ. Yahoo - login M Inbox (3,676) - julio... one.SISU ()Conversations @ iClicker Student - L. School Related Games @ (2) Internet for the ergy * NO @ @ Slideshow nge Tools Add-ons Help Last edit was seconds ago |Background Layout Theme Transition Assumptions with Justification Boiling Soup in Microwave . . Final 60.C L/G G 100 C Temperature (C) 50.C 25 C Initial 0'C S/L C Energy (KJ) bubbles?? + 3Energy * )PHYS 24 - 3/4 Milestone 1 - Inte x G average temperature of a freeze * |4 PHYS 24 LAB - Google Drive | G 109.73 kj ta j - Google Search 1+ (VSP.E315qwjisT)/UYMr/PBTrg3iEe2i170DOU/editslide=id.g 11361906360_0_159 U U GG: The Best Les.. LilkindGuy - Summ.. Yahoo - login M Inbox (3.676) - julio... @ one SISU () Conversations @ iClicker Student - L. School Related Games @ (2) Internet for the. gy @ Slideshow Tools Add-ons Help Last edit was seconds 292 [ Background Layout- Theme Transition Evidence Question #2 Boiling Soup Ice Cooling Soup Cooler, Soup Energy Conservation Equation: = 100 C (-) ( + ) ( + ) ( + ) 1.SOUP -20.C = 60.C Em soup + Emnice + Ein water + Epnice = 0 LICE TI.SOUP 60 C m =0 kg L rice = 0.35 kg L Soup + HO Ice Ice Water Soup 1Emerma 1Emermal T =-20.C m = 0kg L T = 0.C T = 100.C T. = 0.C m, = ? kg L T, = 60 C T = 60.C bubbles
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


