Question
[H;O*). [OH'], and pH Important equations: [H,O) x [OH'] = 1.00 x 1014 pH = -log[H,0] DA solution has a pH = 11.75 .
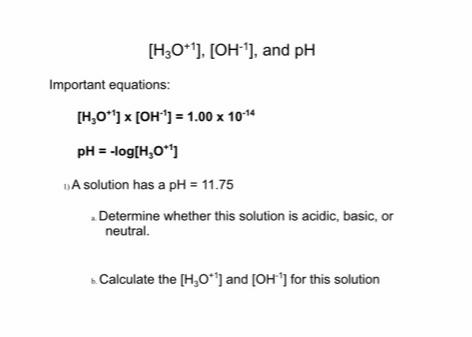
[H;O*"). [OH'], and pH Important equations: [H,O") x [OH'] = 1.00 x 1014 pH = -log[H,0] DA solution has a pH = 11.75 . Determine whether this solution is acidic, basic, or neutral. Calculate the [H,O"] and [OH'] for this solution
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Given H3O OH 1014 and pH of the solution 1175 For neutral point H3O OH so at neutral point H3O ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Discovering Advanced Algebra An Investigative Approach
Authors: Jerald Murdock, Ellen Kamischke, Eric Kamischke
1st edition
1559539844, 978-1604400069, 1604400064, 978-1559539845
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



