Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Homework 1: Material & Energy Balances 1 Example: Suppose 200mol/s of liquid methanol and 30% excess oxygen are fed to a reactor at 25C, a
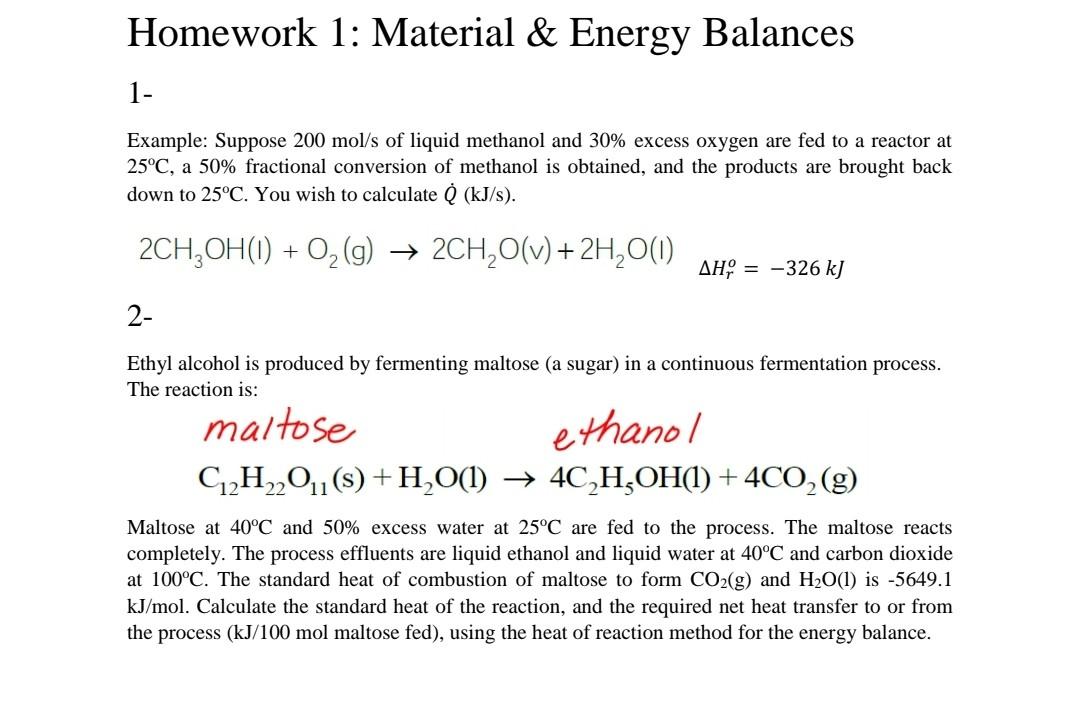
Homework 1: Material \& Energy Balances 1 Example: Suppose 200mol/s of liquid methanol and 30% excess oxygen are fed to a reactor at 25C, a 50% fractional conversion of methanol is obtained, and the products are brought back down to 25C. You wish to calculate Q(kJ/s). 2CH3OH(I)+O2(g)2CH2O(v)+2H2O(I)Hro=326kkJ 2- Ethyl alcohol is produced by fermenting maltose (a sugar) in a continuous fermentation process. The reaction is: maltoseC12H22O11(s)+H2O(l)ethanol4C2H5OH(l)+4CO2(g) Maltose at 40C and 50% excess water at 25C are fed to the process. The maltose reacts completely. The process effluents are liquid ethanol and liquid water at 40C and carbon dioxide at 100C. The standard heat of combustion of maltose to form CO2(g) and H2O(l) is -5649.1 kJ/mol. Calculate the standard heat of the reaction, and the required net heat transfer to or from the process (kJ/100mol maltose fed), using the heat of reaction method for the energy balance
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


