Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How do I do part c? I also don't understand why the (4) & (9) actually mean .0004 & .0009. (2) - You are tasked
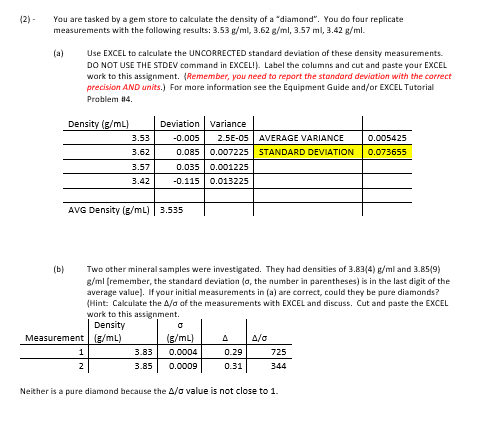

How do I do part c? I also don't understand why the (4) & (9) actually mean .0004 & .0009.
(2) - You are tasked by a gem store to calculate the density of a "diamond". You do four replicate measurements with the following results: 3.53g/ml,3.62g/ml,3.57ml,3.42g/ml. (a) Use EXCEL to calculate the UNCORRECTED standard deviation of these density measurements. DO NOT USE THE STDEV command in EXCEL!). Label the columns and cut and paste your EXCEL work to this assignment. \{Remember, you need to report the standard deviation with the correct precision AND units.) For more information see the Equipment Guide and/or EXCEL Tutorial Problem th4. (b) Two other mineral samples were investigated. They had densities of 3.834g/ml and 3.859 g/ml (remember, the standard deviation o, the number in parentheses) is in the last digit of the average value]. If your initial measurements in (a) are correct, could they be pure diamonds? \{Hint: Calculate the / of the measurements with EXCEL and discuss. Cut and paste the EXCEL work to this assignment. Neither is a pure diamond because the / value is not close to 1 . - Acatic airconia gemsone (which can mimic the look of diarnered density wai cakul aned to be 5.65(9)g/ml. How mafy stafdard deviations different are thew measure-ments from that of the diamond above? FSeE EXCFL. Tuecrial ar Equipment guide for hew to calcalate). Can they be realsticaly separated by deminy? 0Hint: Culculate Alall. Cut and pasee your Evocl calcalaticon molfis insign-mentStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


