Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How do i solve this using the Redlich/Kwong equation? 9. Five mol of calcium carbide are combined with 10mol of water in a closed, rigid,
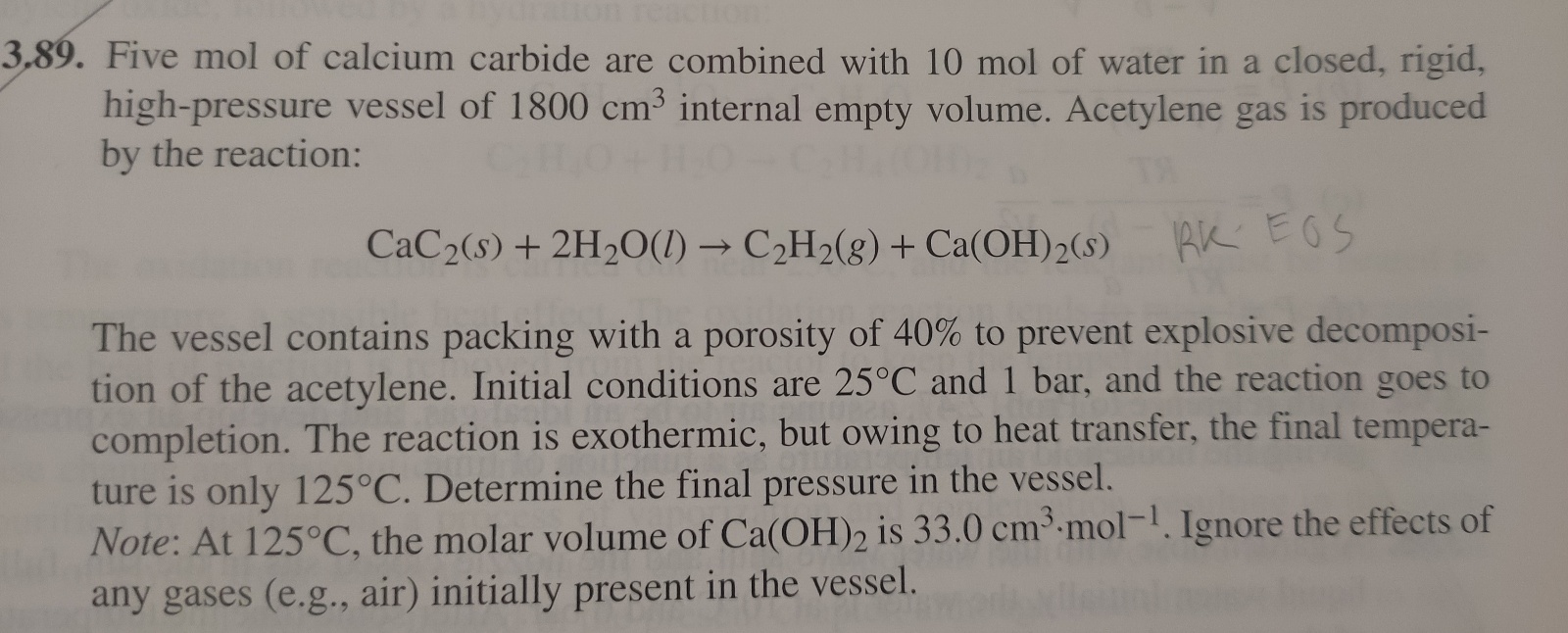
How do i solve this using the Redlich/Kwong equation?
9. Five mol of calcium carbide are combined with 10mol of water in a closed, rigid, high-pressure vessel of 1800cm3 internal empty volume. Acetylene gas is produced by the reaction: CaC2(s)+2H2O(l)C2H2(g)+Ca(OH)2(s)RKEOS The vessel contains packing with a porosity of 40% to prevent explosive decomposition of the acetylene. Initial conditions are 25C and 1 bar, and the reaction goes to completion. The reaction is exothermic, but owing to heat transfer, the final temperature is only 125C. Determine the final pressure in the vessel. Note: At 125C, the molar volume of Ca(OH)2 is 33.0cm3mol1. Ignore the effects of any gases (e.g., air) initially present in the vesselStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


