Question
Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron transfer reaction. Pb2++Zn Species oxidized: Pb+
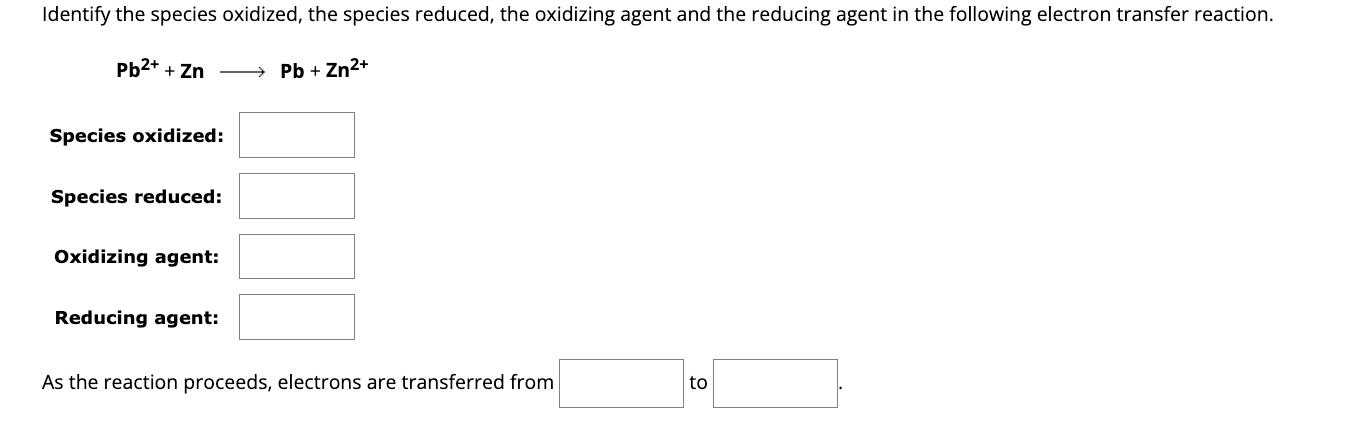
Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron transfer reaction. Pb2++Zn Species oxidized: Pb+ Zn2+ Species reduced: Oxidizing agent: Reducing agent: As the reaction proceeds, electrons are transferred from to
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer Pb 2 ZnPbZn 2 we can identify the species t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organizational Behavior Integrating Individuals Groups And Organizations
Authors: Joseph E. Champoux
4th Edition
0415804647, 9780415804646
Students also viewed these Sociology questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



