Question
Consider diffusion in a spherical particle governed by J cs r with boundary conditions and c t = Ds Cs c r cs =
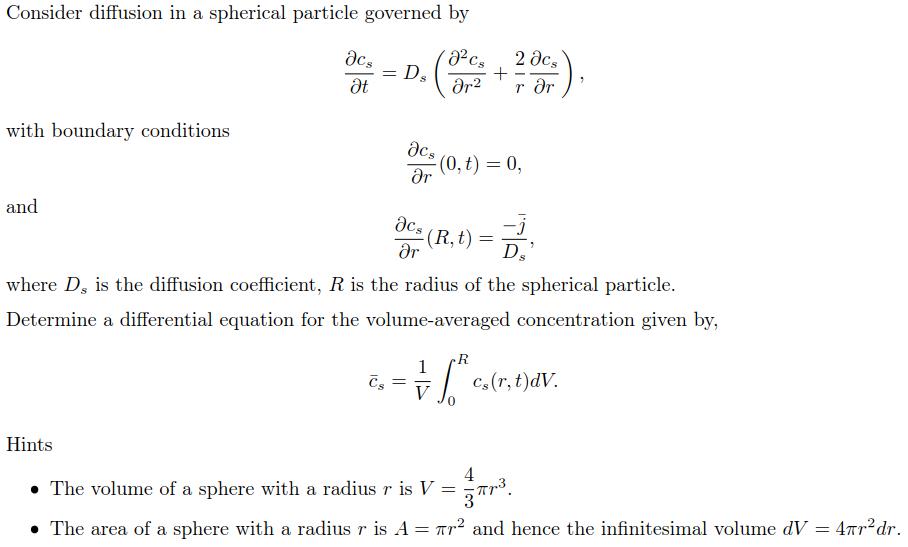
Consider diffusion in a spherical particle governed by J cs r with boundary conditions and c t = Ds Cs c r cs = r + 2 . 2 (R t) = 3/1/ r r (0, t) = 0, where D, is the diffusion coefficient, R is the radius of the spherical particle. Determine a differential equation for the volume-averaged concentration given by, R 1 = = V Cs (r, t)dV. Hints 4 The volume of a sphere with a radius r is V = r. The area of a sphere with a radius r is A = r and hence the infinitesimal volume dV = 4r dr.
Step by Step Solution
3.47 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The given problem involves deriving a differential equation for the volumeaveraged concentration bar...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



