Question
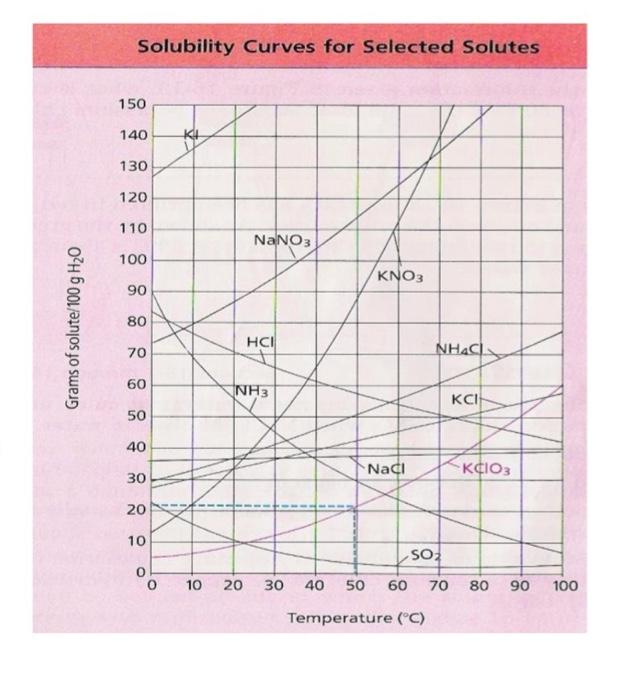
How many grams of KNO, per 100 g of water would be crystallized from a saturated solution as the temperature drops from: Grams of solute/100
How many grams of KNO, per 100 g of water would be crystallized from a saturated solution as the temperature drops from:

Grams of solute/100 g HO Solubility Curves for Selected Solutes 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0 0 NaNO3 HCI NH3 KNO3 Naci NH4C Temperature (C) KCI KIO3 SO 10 20 30 40 50 60 70 80 90 100
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
a b dissoled at 608 113 dissolved at 40es 65 e HNO3 dissolved at 80 C HNO3 dissolved at 20C 3...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of biochemistry Life at the Molecular Level
Authors: Donald Voet, Judith G. Voet, Charlotte W. Pratt
4th edition
470547847, 978-0470547847
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



