Question
How many molecules ofN2O4 are in 76.3 g N2O4? The molar mass of N2O4 is 92.02g/mol. How many molecules of N204 are in 76.3 g
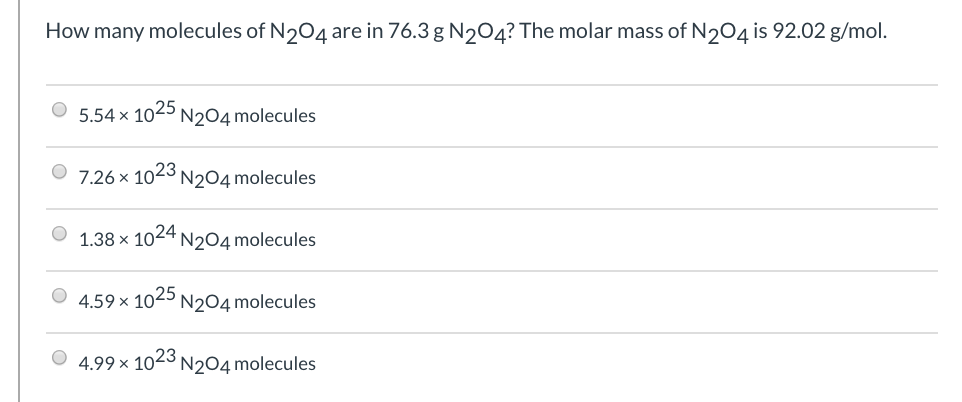 How many molecules ofN2O4 are in 76.3 g N2O4? The molar mass of N2O4 is 92.02g/mol.
How many molecules ofN2O4 are in 76.3 g N2O4? The molar mass of N2O4 is 92.02g/mol.
How many molecules of N204 are in 76.3 g NO4? The molar mass of N2O4 is 92.02 g/mol. 5.54 x 1025 N204 molecules 7.26 x 1023 N204 molecules 1.38 x 1024 N204 molecules 4.59 x 1025 N204 molecules 4.99 x 1023 N2O4 molecules
Step by Step Solution
3.53 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Soln given mass of N204 763 g Molar mass of N204 9202 gm...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



