Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How much energy must be removed from a 94.4g sample of benzene (molar mass =78.11gmol^(-1) ) at 322.0K to solidify the sample and lower the
How much energy must be removed from a
94.4gsample of benzene (molar mass
=78.11gmol^(-1)) at
322.0Kto solidify the sample and lower the temperature to
205.0K? The following physical data may be useful:\
\\\\Delta vap H=33.9kJmol^(-1),\\\\Delta _(fus )H=9.8kJmol^(-1),T_(Sliq)=1.73Jg^(-1)\\\\deg C^(-1),S_(gas )=1.06Jg^(-1)\\\\deg C^(-1)\ Ssol =1.51Jg^(-1)\\\\deg C^(-1),T_(melting )=279.0K,T_(boiling )=353.0K, 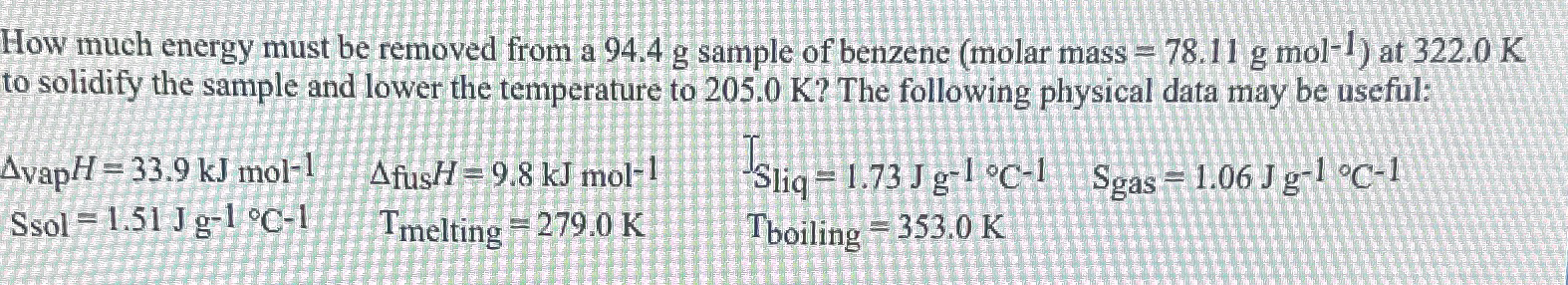
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


