Question
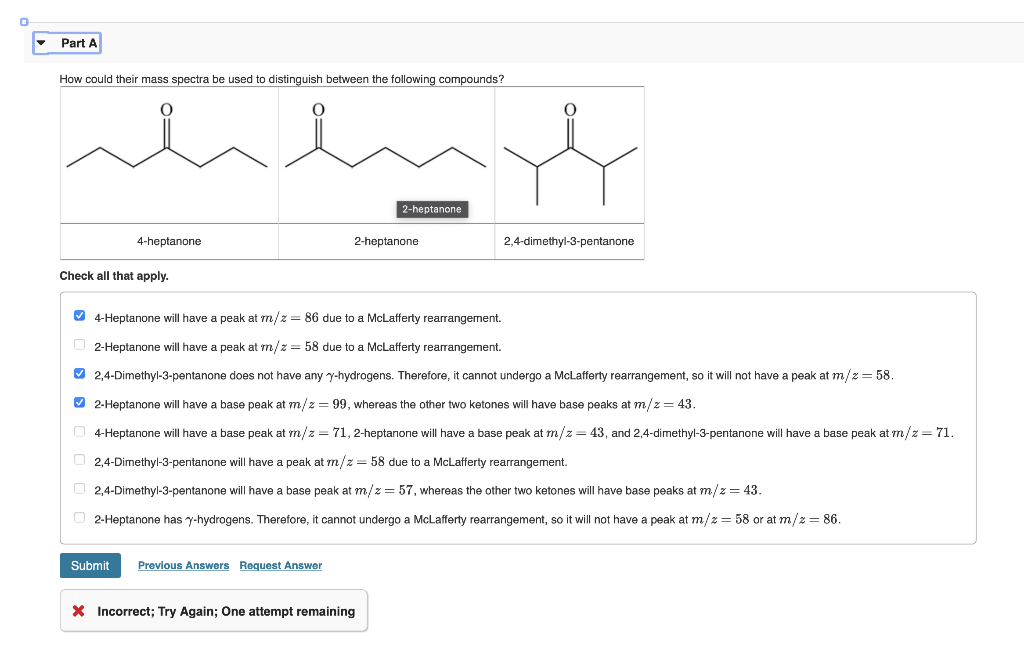
How rnu uld their mase snertra he used to distinnu ush hetumpen the fallowinn rnmnnu unde? all tnat appiy. 4-Heptanone will have a peak at
How rnu uld their mase snertra he used to distinnu ush hetumpen the fallowinn rnmnnu unde?\ all tnat appiy.\ 4-Heptanone will have a peak at
(m)/(z)=86due to a McLafferty rearrangement.\ 2-Heptanone will have a peak at
(m)/(z)=58due to a McLafferty rearrangement.\ 2,4-Dimethyl-3-pentanone does not have any
\\\\gamma -hydrogens. Therefore, it cannot undergo a McLafferty rearrangement, so it will not have a peak at
(m)/(z)=58.\ 2-Heptanone will have a base peak at
(m)/(z)=99, whereas the other two ketones will have base peaks at
(m)/(z)=43.\ 2,4-Dimethyl-3-pentanone will have a peak at
(m)/(z)=58due to a McLafferty rearrangement.\ 2,4-Dimethyl-3-pentanone will have a base peak at
(m)/(z)=57, whereas the other two ketones will have base peaks at
(m)/(z)=43.\ 2-Heptanone has
\\\\gamma -hydrogens. Therefore, it cannot undergo a McLafferty rearrangement, so it will not have a peak at
(m)/(z)=58or at
(m)/(z)=86.\ Incorrect; Try Again; One attempt remaining
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


