Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how to calculate the rate(M/s) and [I] order and [BrO3] order tue first two tables are the data for the clock reaction experiment and here
how to calculate the rate(M/s) and [I] order and [BrO3] order 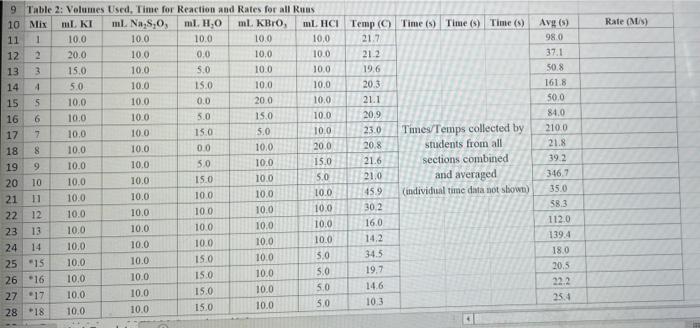
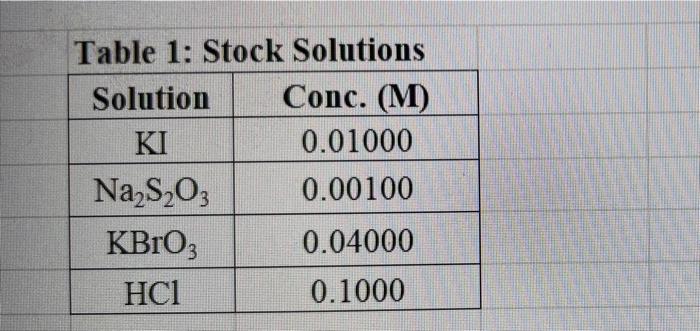
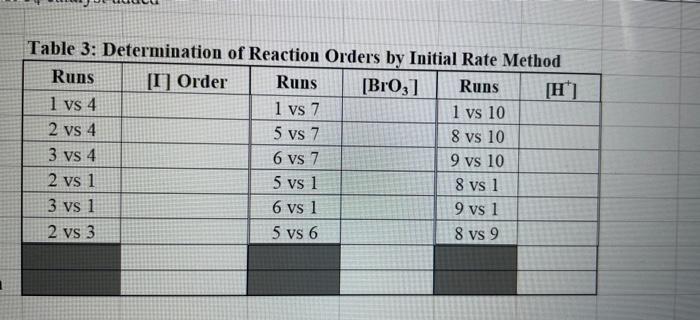



tue first two tables are the data for the clock reaction experiment and here is more information for it 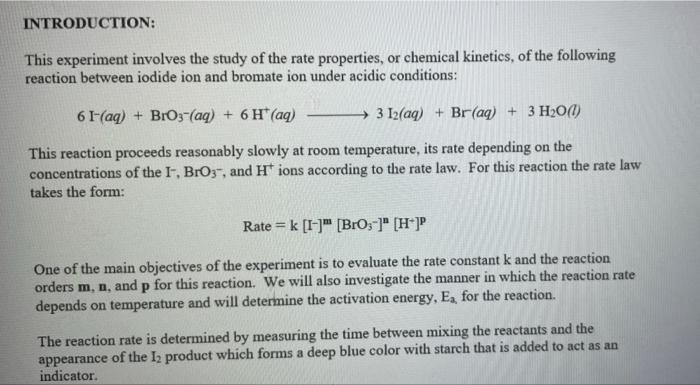
 I want the aswers for table 3 jast one sample calculation for it
I want the aswers for table 3 jast one sample calculation for it
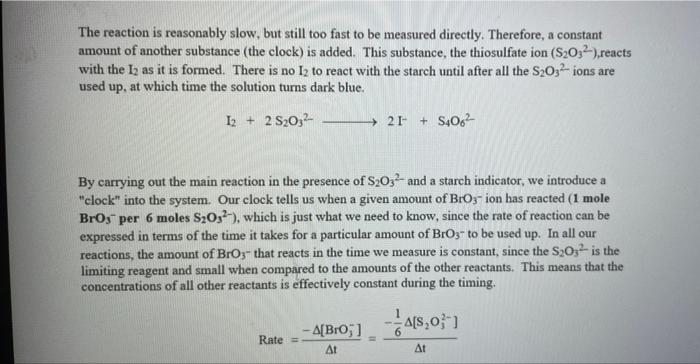
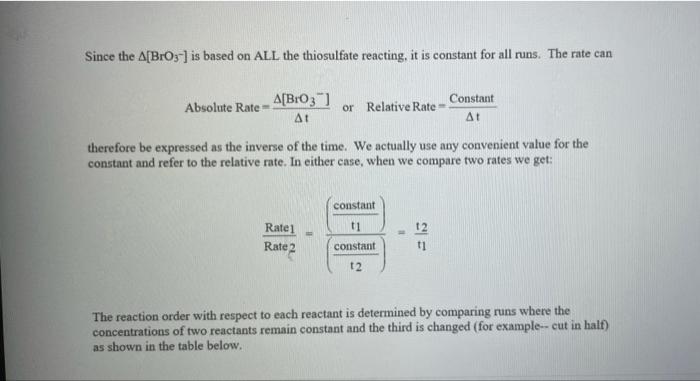
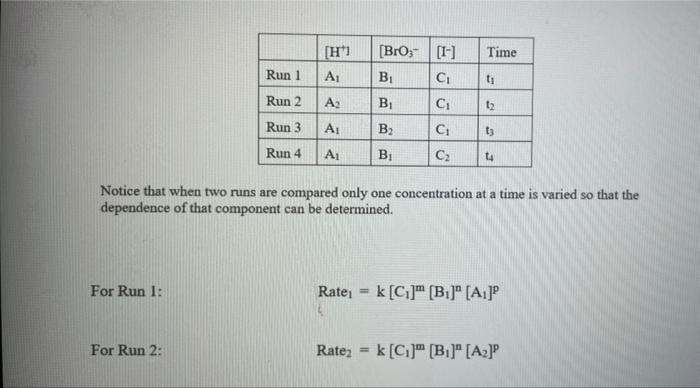
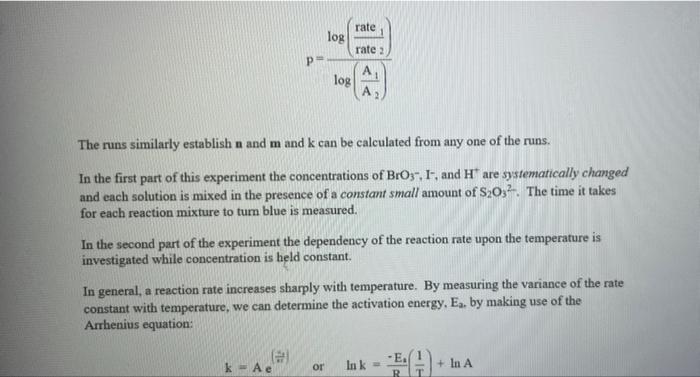
Rate (M) 5.0 9 Table 2: Volumes Used, Time for Reaction and Rates for all Runs 10 Mix mL KI ml. Na,s,o, ml. H0 ml KBIO ml HCI Temp ( Time () Time (>) Time (3) 11 1 10.0 10.0 10.0 10.0 10,0 21.7 12 2 20.0 10.0 0.0 10,0 100 21.2 13 3 15.0 10.0 5.0 10.0 10.0 19.6 14 1 5.0 10.0 15.0 10.0 10.0 20.3 15 5 10.0 10.0 0.0 20.0 10.0 21.1 16 6 10.0 10.0 15.0 10.0 20.9 17 7 10.0 10.0 15.0 5.0 10.0 23.0 Times Temps collected by 18 8 10.0 10.0 10.0 20.0 20.8 students from all 19 9 10.0 10.0 5.0 10.0 15.0 21.6 sections combined 20 10 10.0 10.0 15.0 10.0 5.0 21.0 and averaged 21 11 10.0 10.0 100 10.0 10.0 45.9 (individual time data not show) 22 12 10.0 10.0 100 10.0 10.0 302 23 13 10.0 10.0 10.0 10.0 10.0 16.0 24 14 10,0 10.0 10.0 10.0 10.0 14.2 10.0 25 15 5.0 15.0 10.0 34.5 10.0 10.0 26 16 10.0 15.0 10.0 19.7 5.0 27 17 14.6 10.0 15.0 10.0 10.0 28 18 10.3 50 15.0 10.0 10.0 10.0 AM99 SNN 0.0 Avg (5) 980 37.1 50.8 161.8 50.0 84.0 2100 21.8 39 2 346,7 35.0 38.3 112.0 139.4 18.0 20.5 22.2 25.4 50 Table 1: Stock Solutions Solution Conc. (M) KI 0.01000 Na2S2O3 0.00100 KB103 0.04000 HCl 0.1000 Table 3: Determination of Reaction Orders by Initial Rate Method Runs [I] Order Runs [Br03] Runs 1 vs 4 1 vs 7 1 vs 10 2 vs 4 5 vs 7 8 vs 10 3 vs 4 6 vs 7 9 vs 10 2 vs 1 5 vs 1 8 vs 1 3 vs 1 6 vs 1 9 vs 1 2 vs 3 5 vs 6 8 vs 9 INTRODUCTION: This experiment involves the study of the rate properties, or chemical kinetics, of the following reaction between iodide ion and bromate ion under acidic conditions: 6 1-(aq) + BrO3-(aq) + 6H* (aq) 3 12(aq) + Br(aq) + 3 H20(1) This reaction proceeds reasonably slowly at room temperature, its rate depending on the concentrations of the I-, BrO3-, and Hions according to the rate law. For this reaction the rate law takes the form: Rate = k [I-] [BrO;-)" [H]P One of the main objectives of the experiment is to evaluate the rate constant k and the reaction orders m, n, and p for this reaction. We will also investigate the manner in which the reaction rate depends on temperature and will determine the activation energy, Es for the reaction. The reaction rate is determined by measuring the time between mixing the reactants and the appearance of the Iz product which forms a deep blue color with starch that is added to act as an indicator The reaction is reasonably slow, but still too fast to be measured directly. Therefore, a constant amount of another substance (the clock) is added. This substance, the thiosulfate ion (S202),reacts with the 12 as it is formed. There is no la to react with the starch until after all the S2032-ions are used up, at which time the solution turns dark blue. + 2 S2032- 21 + S40, By carrying out the main reaction in the presence of S2O32- and a starch indicator, we introduce a "clock" into the system. Our clock tells us when a given amount of Brosion has reacted (1 mole Broj per 6 moles S2032), which is just what we need to know, since the rate of reaction can be expressed in terms of the time it takes for a particular amount of BrO3- to be used up. In all our reactions, the amount of BrO3- that reacts in the time we measure is constant, since the S2O3- is the limiting reagent and small when compared to the amounts of the other reactants. This means that the concentrations of all other reactants is effectively constant during the timing. -A[Broj] At A18,0) [" Rate = At Since the A[BrO3-) is based on ALL the thiosulfate reacting, it is constant for all runs. The rate can Absolute Rate A[B103) Constant or Relative Rate - At therefore be expressed as the inverse of the time. We actually use any convenient value for the constant and refer to the relative rate. In either case, when we compare two rates we get: constant 11 12 Ratel Rate 2 constant | = The reaction order with respect to each reactant is determined by comparing runs where the concentrations of two reactants remain constant and the third is changed (for example--cut in half) as shown in the table below. Time [H") A [BrO; [1] B CH Run 1 ti Run 2 A: B 12 C Run 3 A: B2 t3 Run 4 A B C2 14 Notice that when two runs are compared only one concentration at a time is varied so that the dependence of that component can be determined. For Run I: Ratej = k [C] [B]" [A1]P For Run 2: Rate = k[C]" [B]" [A2]P rate log rate 2 P A log A The runs similarly establish n and m and k can be calculated from any one of the runs. n In the first part of this experiment the concentrations of BrO3-, 1-, and Hare systematically changed and each solution is mixed in the presence of a constant small amount of S2032-. The time it takes for each reaction mixture to turn blue is measured. In the second part of the experiment the dependency of the reaction rate upon the temperature is investigated while concentration is held constant. In general, a reaction rate increases sharply with temperature. By measuring the variance of the rate constant with temperature, we can determine the activation energy, E. by making use of the Arrhenius equation: k - Ae or Ink -E. R + In A 



 I want the aswers for table 3 jast one sample calculation for it
I want the aswers for table 3 jast one sample calculation for it Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


