Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how to calculate % yield, crude yield, purified yield. please show all work Synthesis of t-pentyl chloride: Theoretical yield: 0.0911mol1mol1106.59=9.7g Continued from Page Results for
how to calculate % yield, crude yield, purified yield. please show all work 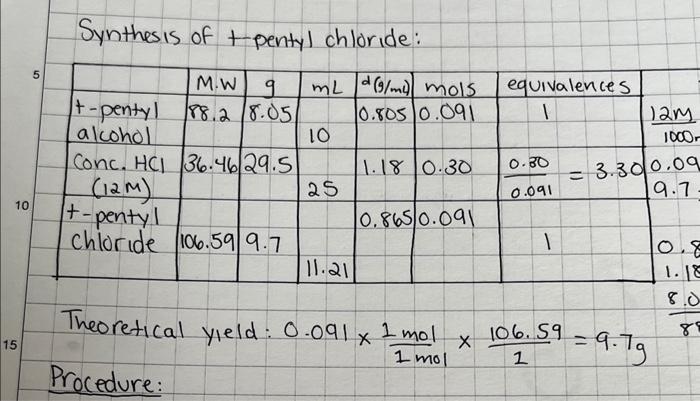
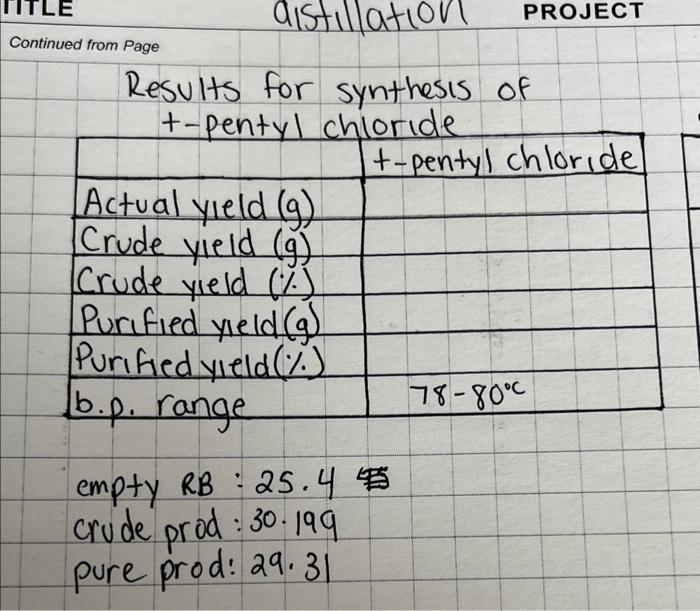
Synthesis of t-pentyl chloride: Theoretical yield: 0.0911mol1mol1106.59=9.7g Continued from Page Results for synthesis of t-pentyl chloride \begin{tabular}{|l|l|} \hline & \\ \hline Actual yield (g) & \\ \hline Crude yield (g) & \\ \hline Crude yield (%) & \\ \hline Purified yeeld (g) & \\ \hline Purified yield (%) & \\ \hline b.p. range & 7880c \\ \hline \end{tabular} empty RB : 25.4 crude prod: 30.199 pure prod: 29.31 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


