Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how to solve by polymath? Q1: The dehydration of butanol is carried out over a silica-alumina catalyst at 680 K. CH.CH,CH.CH,OH CH CH=CHCH, +H,0 The
how to solve by polymath? 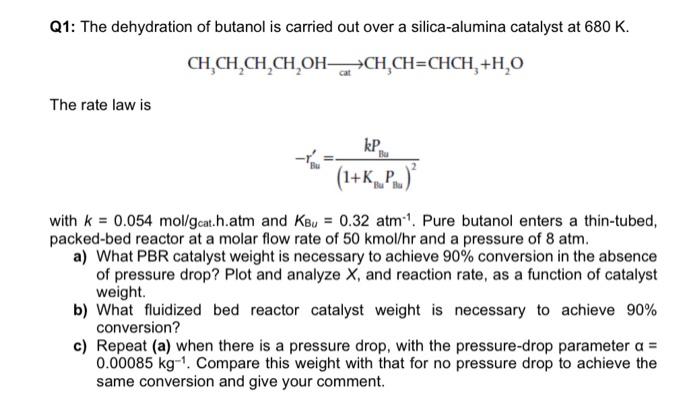
Q1: The dehydration of butanol is carried out over a silica-alumina catalyst at 680 K. CH.CH,CH.CH,OH CH CH=CHCH, +H,0 The rate law is Bu kp. -V (1+K_P..) with k = 0.054 mol/gcat.h.atm and Keu = 0.32 atm 1. Pure butanol enters a thin-tubed, packed-bed reactor at a molar flow rate of 50 kmol/hr and a pressure of 8 atm. a) What PBR catalyst weight is necessary to achieve 90% conversion in the absence of pressure drop? Plot and analyze X, and reaction rate, as a function of catalyst weight. b) What fluidized bed reactor catalyst weight is necessary to achieve 90% conversion? c) Repeat (a) when there is a pressure drop, with the pressure-drop parameter a = 0.00085 kg-1. Compare this weight with that for no pressure drop to achieve the same conversion and give your comment 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


