Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How to solve t - Butyl alcohol ( TBA ) is an important octane enhancer that is used to replace lead additives in gasoline. TBA
How to solve
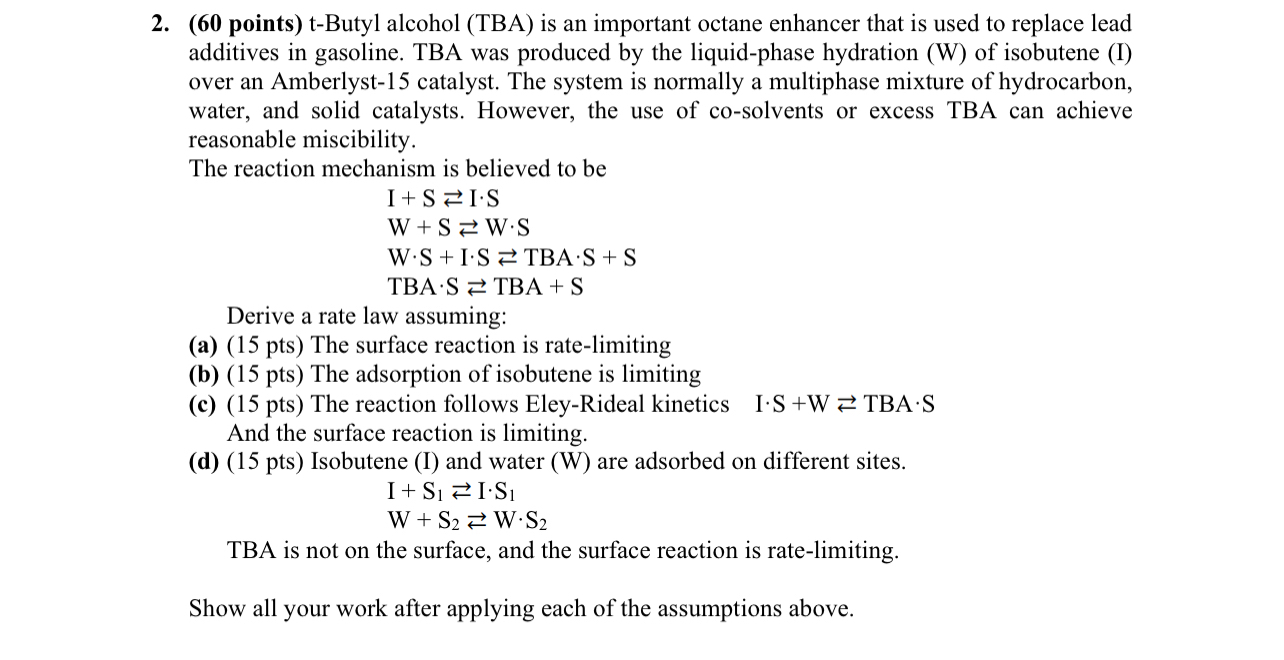
tButyl alcohol TBA is an important octane enhancer that is used to replace lead additives in gasoline. TBA was produced by the liquidphase hydration W of isobutene I over an Amberlyst catalyst. The system is normally a multiphase mixture of hydrocarbon, water, and solid catalysts. However, the use of cosolvents or excess TBA can achieve reasonable miscibility.
The reaction mechanism is believed to be
TBA
Derive a rate law assuming:
a pts The surface reaction is ratelimiting
b pts The adsorption of isobutene is limiting
c pts The reaction follows EleyRideal kinetics
And the surface reaction is limiting
d pts Isobutene I and water W are adsorbed on different sites.
TBA is not on the surface, and the surface reaction is ratelimiting
Show all your work after applying each of the assumptions above.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


