Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how to solve this? pictutes of additional information from lab included. Imagine that you started from 5.00 grams of BaCl2.2H2O, which is your limiting reactant.
how to solve this? pictutes of additional information from lab included. 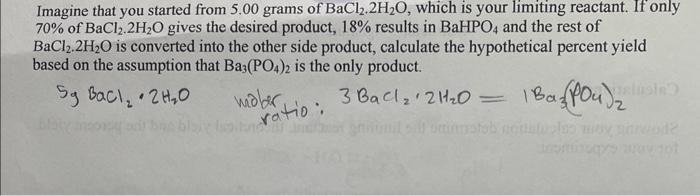
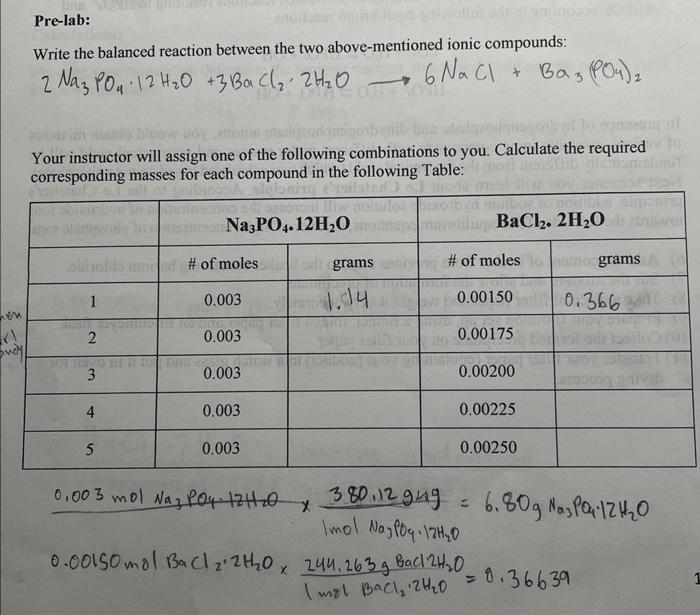
Imagine that you started from 5.00 grams of BaCl2.2H2O, which is your limiting reactant. If only 70% of BaCl2.2H2O gives the desired product, 18% results in BaHPO4 and the rest of BaCl2.2H2O is converted into the other side product, calculate the hypothetical percent yield based on the assumption that Ba3(PO4)2 is the only product. 5gBaCl2H2Owobr3BaCl22H2O=1Ba3(PO4)2 Write the balanced reaction between the two above-mentioned ionic compounds: 2Na3PO412H2O+3BaCl22H2O6NaCl+Ba3(PO4)2 Your instructor will assign one of the following combinations to you. Calculate the required corresponding masses for each compound in the following Table 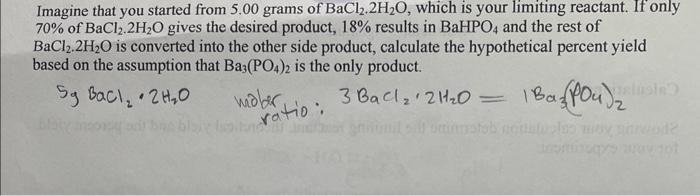

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


