Question: How would I construct aninformative speechfor Dr. John L. LaMattina, President, Global Research and Development, that will be delivered telephonically to shareholders at the quarterly
How would I construct aninformative speechfor Dr. John L. LaMattina, President, Global Research and Development, that will be delivered telephonically to shareholders at the quarterly earnings call. The shareholders will be looking to the President to present abrighter outlook for the company that will include improved cash flows, future profit projections, and moderate growth.
Using the case study below:
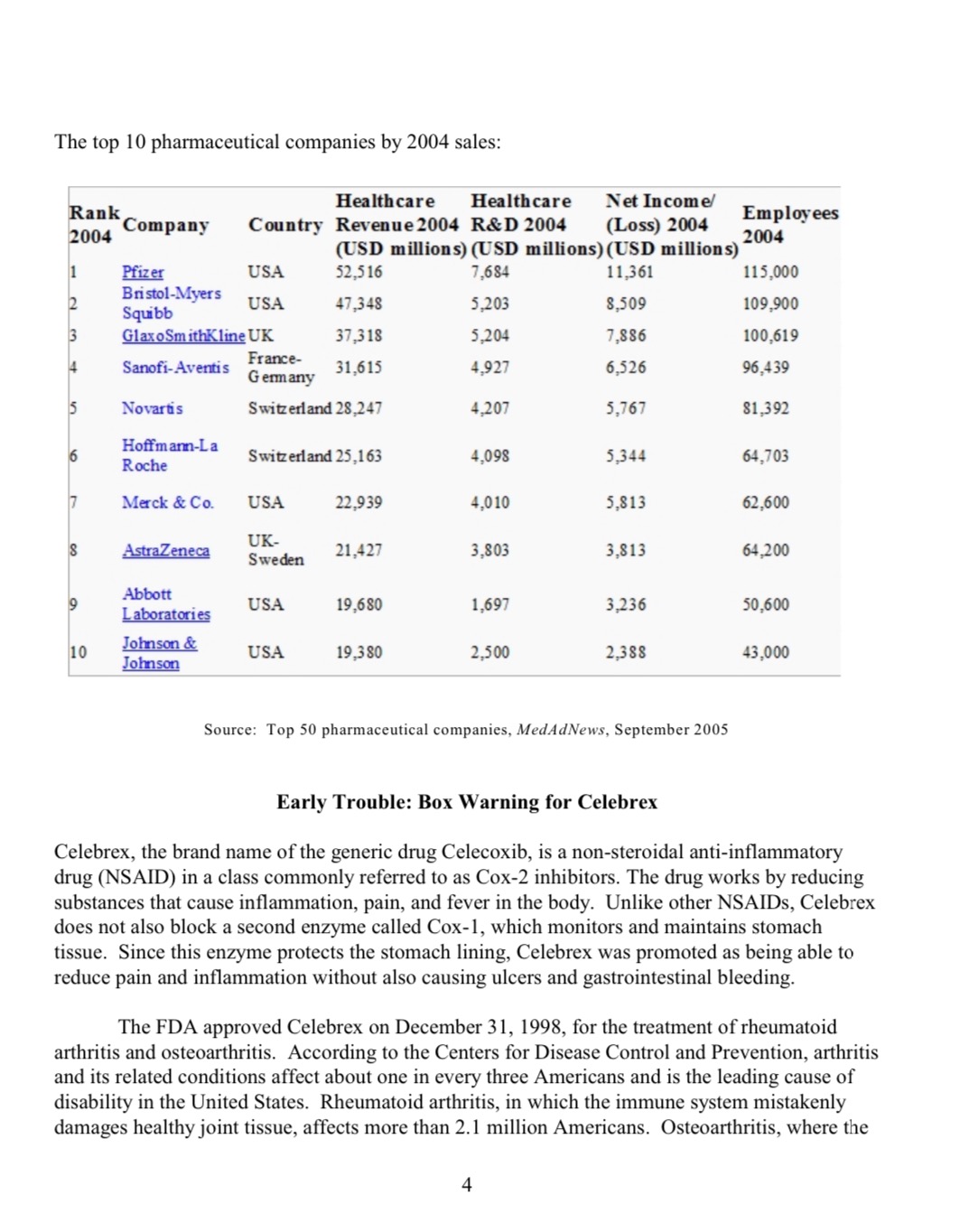
Pfizer Inc.:The Torcetrapib Failure and the Future of the World's Largest Drug ManufacturerIt was Saturday December 2, 2006, and Dr. John L. LaMattina, Pfizer's chief scientist heard the alarm clock next to his bed go off. He awoke and got in the shower. As he turned on the hot water, LaMattina let his mind wander, looking forward to the weekend. It was 7:00 a.m., Eastern Standard Time.The ringing telephone quickly brought him back to reality. The news coming from the other end of the phone line was devastating. Torcetrapib, Pfizer's most promising drug in clinical trials, intended to treat coronary heart disease, actually caused an increase in deaths and heart problems. During the clinical trial, 82 patients taking a combination of Torcetrapib and Lipitor had died, compared to 51 patients in the same trial taking Lipitor alone. Dr. LaMattina called Pfizer's CEO, Jeffery Kindler and by 8:00 a.m. senior leaders were going over the results in a conference call.Within a few hours of the conference call, Pfizer put a stop to the Torcetrapib clinical trial and began telling its trial investigators to stop giving the drug to trial patients. By 9:00 p.m., Pfizer announced that it was pulling the plug on the trial and Torcetrapib development as a whole. "Mr. Kindler," according to a reporter, "said he was surprised and disappointed at the findings. Torcetrapib's failure highlights the risks that drug makers face as they try to develop new and important medicines. This is a very high-risk business, the company is financially strong and expects to report higher profit in both 2007 and 2008, mainly due to cost-cutting" The failure of Torcetrapib came on the heels of the Bextra and Celebrex problems that forced Pfizer to pull Bextra off the market and to black box label Celebrex. Pfizer pledged to cut $4 billion in costs by 2008 and announced it was going to lay off 7,800 employees and close several research centers, mostly in Europe. The company's stock was down 11% on Monday following the announcement. Pfizer shares traded a volume of 235 million shares, more than2For the world's largest drug company the future was uncertain. One thing was for sure, though. The future would bring change. But would that change be best for the company, its customers and patients, or its investors?Pfizer HistoryPfizer, the world's largest pharmaceutical company was founded in 1849 as a chemical company. From 1849 to 1899, the company mainly produced products such as citric acid, camphor, cream of tartar, borax and iodine. Between 1899 and 1950, Pfizer became a leader in fermentation and began to mass produce penicillin. From 1951 to 1999, Pfizer focused heavily on international expansion and aggressive growth through the introduction of new drugs such as Vibramycin, a once-a-day broad-spectrum antibiotic. Other products included Feldene, one of the largest- selling prescription anti-inflammatory medications in the world, Glucotrol for diabetes, Zoloft for depression, Norvasc for control of hypertension, and Zithromax for respiratory and skin3Today, Pfizer manufactures pharmaceutical medications. The company is, in it's own words, ". . . dedicated to better health and greater access to health care for people and their valued animals. Every day, approximately 87,000 colleagues in more than 150 countries work to discover, develop, manufacture and deliver quality, safe and effective prescription medicines to patients."4The Process of Developing Pharmaceutical DrugsNew drugs are discovered by aggressive research investments that lead to either the rational development of a drug or simple, accidental discovery of medical potential in certain compounds. The modern approach has been to study the effects on the molecular and physiological level by disease and infection in order to target specific entities.The development process for drugs is cost intensive and has a small rate of success. Heavy investments are required in areas such as pre-clinical development, clinical trials and safety2seven times normal trading volume.infections.rights to Lipitor. In 2002, Pfizer merged with Pharmacia to become the largest pharmaceutical company in the world. This merger involved the rights to Celebrex, a Cox-2 selective inhibitor collectively marketed by Pfizer and Pharmacia.In 2000, Pfizer merged with Warner-Lambert which allowed Pfizer to acquire fullmonitoring to determine the safety of a compound. Pre-clinical development usually involves animal testing to estimate the safe starting dose of the drug for human clinical trials.Clinical Trial PhasesPhase I involves a small group of healthy volunteers (usually about 30). The initial phase is designed to assess safety and tolerability of a therapy. A full-time medical staff observes patients until several half-lives of the drug have passed. This phase also includes tests on the proper dosage for human consumption. The very first patient is given a small dose of the drug and if all goes well, the next patient is given a slightly higher dose.Phase II trials are performed on larger groups (30 to 50 patients) and attempt to assess the medical benefits of the drug. About seventy percent of drugs make it form Phase I to Phase II. However, many drugs fail in phase 2 due to the discovery of poor efficacy or negative side effects.Phase III trials aim to compare the new treatments with the best existing treatment and are usually much larger than Phase II. This phase involves many different trial centers that can be in different countries and these trials are often randomized. During this phase, drug companies attempt to further confirm the medical benefits of the drug and its safety. Due to the large patient group and the difficult trials that must be run, this phase is usually the most capital- and time- intensive. Trial procedures and results are compiled into a New Drug Application that also contains manufacturing procedures, formulation, and shelf life. This application is submitted to the government regulatory agency, such as the FDA in the U.S., for approval. Once approval is granted, the pharmaceutical company receives the right to market the drug to consumers.Phase IV takes place after a drug is shown to work and regulatory approval has been granted. The purpose of this phase is to find out more about side effects, long-term risk and benefits, and drug efficacy now that it is being used on a much larger scale.Safety monitoring, which occurs throughout the development process, continues after the drug is on the market; the pharmaceutical company provides ongoing monitoring as well as technical support. This is designed to detect long-term adverse effects due to timescale that are impossible to achieve during clinical trials. Detection of adverse effects may lead to the withdrawal or restrictions of a drug.For the first time ever, in 2006, global spending on prescription drugs topped $600 billion, even as growth slowed somewhat in Europe and North America. Sales of prescription medicines worldwide rose 7 percent to $602 billion, according to IMS health, a pharmaceutical information and consulting company. The United States still accounts for most with $252 billion in annual sales, growing 5.7 percent. Emerging markets such as China, Russia, South Korea, and35Lipitor remains the best-selling drug in the world with sales of $12.9 billion.Mexico outpaced that market, growing a huge 81 percent.Pfizer's cholesterol controlling drug,The top 10 pharmaceutical companies by 2004 sales: Source: Top 50 pharmaceutical companies, MedAdNews, September 2005Early Trouble: Box Warning for CelebrexCelebrex, the brand name of the generic drug Celecoxib, is a non-steroidal anti-inflammatory drug (NSAID) in a class commonly referred to as Cox-2 inhibitors. The drug works by reducing substances that cause inflammation, pain, and fever in the body. Unlike other NSAIDs, Celebrex does not also block a second enzyme called Cox-1, which monitors and maintains stomach tissue. Since this enzyme protects the stomach lining, Celebrex was promoted as being able to reduce pain and inflammation without also causing ulcers and gastrointestinal bleeding.The FDA approved Celebrex on December 31, 1998, for the treatment of rheumatoid arthritis and osteoarthritis. According to the Centers for Disease Control and Prevention, arthritis and its related conditions affect about one in every three Americans and is the leading cause of disability in the United States. Rheumatoid arthritis, in which the immune system mistakenly damages healthy joint tissue, affects more than 2.1 million Americans. Osteoarthritis, where the4cartilage in the joints of the hands, hips, or knee deteriorates from normal use, affects most6people over sixty.After the withdrawal of Vioxx from the market in September 2004, Celebrex enjoyed a significant increase in sales. However, in December 2004, a colon cancer prevention trial conducted by the National Cancer Institute found patients taking a high dose of Celebrex (400mg to 800mg) had increased cardiovascular risk compared with placebos. The study, called the Adenoma Prevention with Celecoxib (APC) was cut short because results demonstrated patients taking Celebrex had almost twice the risk of a major cardiovascular event as people on placebos.On the other hand, a large Alzheimer prevention trial called ADAPT found the over-the- counter drug Aleve demonstrated an increase in cardiovascular risk (CV) compared to placebo8In April 2005, the FDA issued a press release, saying "we believe that it is reasonable to conclude there is a 'class effect' for increased cardiovascular risk for all NSAIDs." The FDA9Responding to the FDA black box label, Pfizer agreed to fund a study directed by the Cleveland Clinic that compares Celebrex with traditional anti-inflammatory drugs such as Aleve and Ibuprofen. The study, called PRECISION, includes over 20,000 high-risk patients and is scheduled to be complete in 2010. Ultimately, this study will help determine if any cardiovascular risks are a result of the Celebrex drug.Withdrawal of BextraThe FDA approved Bextra, the brand name version of the generic drug valdecoxib, on November 16, 2001. The drug is also part of the Cox-2 inhibitor family and its intended use was to relieve symptoms of osteoarthritis, rheumatoid arthritis, and painful menstrual cycles. However, bad news would come quickly for Pfizer regarding Bextra's side effects.In 2004, the American Heart Association issued a report indicating patients using Bextra while recovering from heart surgery were 2.19 times likely to suffer a stroke or heart attack than those taking placebos. Pfizer acknowledged these increased risks but a New York Times article5but a high dose of Celebrex revealed no additional risks.David Graham of the Office of Drug Safety at the FDA and a meta-analysis published in the Journal of the American Medical Association did not find increased cardiovascular risks in Celebrex patients compared to those taking placebos. Neither of the Celebrex studies was conducted or sponsored by Pfizer. The APC trial and the Vioxx study led to speculation of CV risk only in Cox-2 drugs. Sales of Celebrex began to fall dramatically after these reports.mandated all prescription NSAIDs carry a boxed warning for cardiovascular risk.asked Pfizer to halt direct advertising to consumers and encourage doctors to seek alternatives with its patients. By September 2005, Celebrex's sales fell by 45% over previous year's levels.In addition, a large study conducted byThe FDA also 107published in November 2004 stated that scientists outside the company had evidence that Bextra's problems may affect wider groups of patients.Pfizer reviewed information regarding the cardiovascular profile of Bextra. The company stated that available clinical information on Bextra suggested no increased risk of cardiovascular events in patients treated for osteoarthritis and rheumatoid arthritis. This information was based on analyses of a clinical trial database of nearly 8,000 patients treated with Bextra for durations11However, in April 2005, the U.S. Food and Drug Administration announced that it has asked Pfizer to voluntarily withdraw Bextra from the U.S. market. Pfizer agreed to suspend sales and marketing of Bextra in the U.S. The FDA stated that it "has concluded that the overall risk versus benefit profile of Bextra is unfavorable. This conclusion is based on the potential increased risk for serious CV events and the fact that Bextra has not been shown to offer any unique advantages over the other available NSAIDs."12What are HDL and LDL?Cholesterol is a fatty substance that is an important part of the cell membrane in the body of animals and in the blood circulation of people. The main sources of cholesterol for humans are dietary intake and internal liver production. The liver has a dual function as cholesterol regulator, as it can take cholesterol out of the blood stream or put it back in as necessary. Usually after a meal, cholesterol is absorbed by the intestines into the blood circulation and packaged inside a protein coat called a chylomicron. After a meal, the liver removes chylomicrons from the blood circulation and in between meals it releases chylomicrons back into blood circulation. The main vessel through which the liver performs this transfer is called cholesterylester transfer protein (CETP).Cholesterol consists of two parts: LDL (low density) cholesterol, also known as bad cholesterol because it is directly associated with increased risk of coronary heart disease. LDL lipoproteins deposit cholesterol on the artery walls forming a hard, thick substance called plaque. With time, plaque builds up and causes a narrowing of the arteries, also known as atherosclerosis. Atherosclerosis can then result in blood clots, stroke or heart attacks. HDL (high density) cholesterol is called good cholesterol because its particles remove plaque from the arteries and dispose of it through the liver, therefore reducing the risk of coronary heart diseases. There are two other kinds of lesser mentioned cholesterol VLDL (very low density) and IDL (intermediate density). Total cholesterol is measured as the sum of all four levels. Heredity and diet are the two main factors of levels of cholesterol in the human body. Currently most cholesterol drugs, such as Lipitor, target LDL as the main way of minimizing risk of disease. However, another hypothesis - elevating HDL - is suggested as a more effective way of controlling cholesterol and lowering risk of coronary heart diseases.6ranging from six to 52 weeks.The Torcetrapib TimelineIn 1990, Pfizer set out to develop a novel medicine aimed at significantly reducing the risk of heart disease in humans. A scientific paper in the New England Journal of Medicine reported that a group of Japanese patients who were lacking the CETP protein had high levels of HDL and low incidence of coronary artery disease. Pfizer began researching the issue and developed a promising chemical compound known as CP529, 414 designed to increase levels of HDL by blocking CETP's ability to transfer bad cholesterol from the liver into the blood stream. This compound was identified in 1993, modified in 1994, and came to be known as Torcetrapib. In 1999, first human trials were initiated to demonstrate how Torcetrapib works in the human body. A year later, Pfizer began Phase II trials which were larger in scale and were aimed at testing further efficacy and safety of the drug. Torcetrapib itself proved inadequate in lowering LDL levels in the early trials so it was suggested to combine it with atorvastatin in order to improve its efficacy. Torcetrapib was therefore combined with Lipitor during the larger scale clinical trials.In 2003, Pfizer initiated Phase III trials to test the drug's effectiveness against disease. At the same time it invested over $800 million for testing and further research. In 2004, Pfizer began enrollment for Illuminate, the morbidity and mortality clinical trial for its Torcetrapib/atorvastatin drug combination. At this time, Pfizer built a $90 million expansion plant in Loughbeg, Ireland in order to produce Torcetrapib. The new facility would employ 40 employees and it had the latest in analytical technology, on-line sensing instruments, and a wide range of novel manufacturing technologies. "If we prove our hypothesis, torcetrapib/atorvastatin has the potential to benefit millions of lives around the world," said Dr. John L. LaMattina, president of Pfizer Global Research and Development. "Nothing is certain except our huge investment. Even if this fails as a new medicine, we will have advanced scientific understanding in this area."13Illuminate Clinical TrialsIn 2004, Pfizer initiated a large scale trial (Phase III) of its Torcetrapib/atorvastatin drug combination with15,000 patients participating in the trial. The large trial was named Illuminate. (Investigation of Lipid Level management to Understand its iMpact IN ATherosclerotic Events) It was global in scale and several research centers were involved in administering it. The test was also double blind. "Double blind means that one researcher allocates a series of numbers to 'new treatment' or 'old treatment.' The second researcher is told the numbers, but not what they have been allocated to. Since the second researcher does not know, they cannot possibly tell the patient, directly or otherwise, and cannot give in to patient pressure to give them the new treatment. In this system, there is also often a more realistic distribution of sexes and ages of patients. Therefore double-blind (or randomized) trials are preferred, as they tend to give the most accurate results."14 The trial also included what is known as parallel group evaluation, which means that the 15,000 patients participating in the study were divided in two equal groups7of approximately 7,500 each. One group was given the Torcetrapib/atorvastatin combination while the other group was given the atorvastatin alone (Lipitor).On December 2, 2006, Pfizer was told by the independent Data and Safety Monitoring Board (DSMB) for the ILLUMINATE trials that the DSMB was recommending termination of the trial. Pfizer issued a public announcement that it was terminating all ongoing torcetrapib15generally showing that Torcetrapib/atorvastatin combination significantly increased HDL cholesterol by 55 to 60 percent and lowered LDL cholesterol by 10 to 15 per cent over atorvastatin alone (leading to a combined reduction in LDL cholesterol of 50 to 60 per cent)."16Clinical Trial StoppedAfter learning the news of the DSMB recommendation from Pfizer's head of R&D about 7:00a.m. on Saturday, December 2, CEO Jeff Kindler quickly assembled the senior leadership team atPfizer's New York headquarters, along with other key personnel from all affected areas - fromresearch and development, to scientific and regulatory affairs to communications. The leadershipquickly made the decision that in the interests of patients' safety and welfare, to end all clinical17The Data Safety Monitoring Board recommended ending the trials and Pfizer followed along with this recommendation putting an end to Illuminate and to the entire Torcetrapib research. The main reason for ending the ILLUMINATE trials was that the group of patients taking the Torcetrapib/atorvastatin combination had 82 deaths compared to 51 deaths in the group taking Lipitor alone. The results had enough statistical significance to warrant the end of the clinical trials. "Pfizer, Inc said that in the interests of patient safety it is stopping all Torcetrapib clinical trials and that it has informed the Food and Drug Administration. The company is in the process of notifying all clinical investigators in the program as well as other regulatory authorities."18Pfizer's communication team is organized into four functions within Worldwide Communications; a Senior Vice President heads the department. The names and corresponding responsibilities include:? Communications Strategy, Media Relations & Issues Management: Divisional and geographic communications oversight, advise and counsel; centralized strategy and issues management; media relations aligned with corporate, product and research functions.8The first doubts were raised about Torcetrapib because the patient group that took it showed signs of higher systolic blood pressure when compared to those who were taking Lipitor alone. "Overall lipid results from all the completed trials were positive,trials later on December 2, 2006.trials immediately. ? Strategic Planning, Management & Operations: Planning and management of signature company and CEO events; opinion and messaging research; corporate advertising & sponsorship; day-to-day organization of the department.? Colleague Communications: Delivering a comprehensive internal communications strategy to drive colleague engagement. Includes production and management of Pfizer's intranet site and electronic newsletter.? CEO & Executive Communications: Speechwriting, presentation development, writing support and counsel for the CEO and Pfizer senior leadership team, including: platform development, speaking engagements and other public interactions.Pfizer's plan was to execute a communications strategy to ensure that it reached all of its critical audiences - including patients, the medical establishment, regulatory agencies, shareholders, the media, the financial community and colleagues. It was of the utmost importance to reach all of these audiences.The company needed to inform more than 25,000 patients around the world who were taking part in multiple clinical trials, to cease taking their study medication as soon as possible. Pfizer also wanted to reinforce for all patients who take its medicines, those already on the market as well as those in clinical trials, that patient safety is Pfizer's first and foremost concern and responsibility.The Pfizer medical team immediately began outreach to hundreds of clinical investigators and numerous regulatory authorities around the world to notify them of the decision. Later, the company worked with physicians presenting data about Torcetrapib at major medical meetings to coordinate the communications about Torcetrapib. All of these efforts served to enhance Pfizer's relationship with the medical community and advance Pfizer's reputation of putting patients first.Pfizer also reached out to the media to provide as much information as possible so that the news coverage and subsequent public opinion would be as balanced and well-informed as possible. The company felt it was necessary to update members of the media with whom it has good working relationships and who had covered Torcetrapib throughout its development to let them know exactly what Pfizer had learned. The company also made its communications team, medical experts and executives available to them to provide clarity. A press release was issued that Saturday evening at 9:00 p.m. to announce the discontinuation of the clinical trials program. Designated spokespeople were made available and quoted in several news outlets in the coming days, as was the CEO, who also appeared live on select television news shows in the days following.The company also informed the financial community, including investors and analysts, to update them on this important company development. CEO Jeff Kindler sent an e-mail to all9 Pfizer colleagues that night to explain the situation. To supplement the e-mail, he also taped a video segment further explaining the news, which was made available for colleagues to view on the company intranet.The Financial ImplicationsFinding the medical reasons why Torcetrapib failed is only one of the concerns of Pfizer. While the company will not likely face product liability for the deaths (they happened during consented clinical trials) the company has a difficult road ahead. Torcetrapib was thought to be the next great breakthrough in curing coronary heart disease. Some estimates claim that first year of sales for the drug would have surpassed twelve billion dollars and would have been a very good replacement for revenues that would be lost from Lipitor losing patent protection. "I'm terribly disappointed," said Dr. Steven E. Nissen, chairman of cardiovascular medicine at the Cleveland Clinic and lead investigator of an earlier torcetrapib clinical trial. "This drug, if it worked, would probably have been the largest-selling pharmaceutical in history."19In the next few years Pfizer will lose patent protection on some of its most successful drugs. Zoloft lost its patent protection and became subject to generic competition in 2006; Norvasc lost its patent in 2007, Aricept and Lipitor will follow suit 2010 and 2011 respectively. When a drug becomes generic, a company must cut sales prices to compete against those that produce cheaper versions, which results in much significantly lower revenues.Currently Pfizer has more clinical trials and research programs than at any other time in its history. It has a broad set of promising new therapies in oncology, cardiovascular disease, obesity, schizophrenia, rheumatoid arthritis, HIV infection and Alzheimer's disease, among others. The company is also increasing R&D investment in areas that look especially promising, such as biotherapeutics. Pfizer plans to launch four new internally developed products each year, starting in 2011 and aims to launch two new externally sourced products each year starting in 2010.The company is also in the midst of a large-scale cost reduction initiative across the entire company, and is focused on establishing a more flexible cost structure. These activities include staff reductions, site closures, and increased outsourcing and procurement savings.In an industry in which the average development of a drug can take as long as twelve years, where does Pfizer find itself in the next five to ten years? What can the largest drug manufacturer in the world do to offset the loss of patents and future revenue while potential new products are still in the research pipeline and the company continues to face investor pressure for growth and returns? Is laying off workforce and cutting costs a viable strategy for a company where research and development is crucial to its survival? With analysts predicting shares bottoming at $20.00/share what can Pfizer do to regain investor confidence?








Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


