Answered step by step
Verified Expert Solution
Question
1 Approved Answer
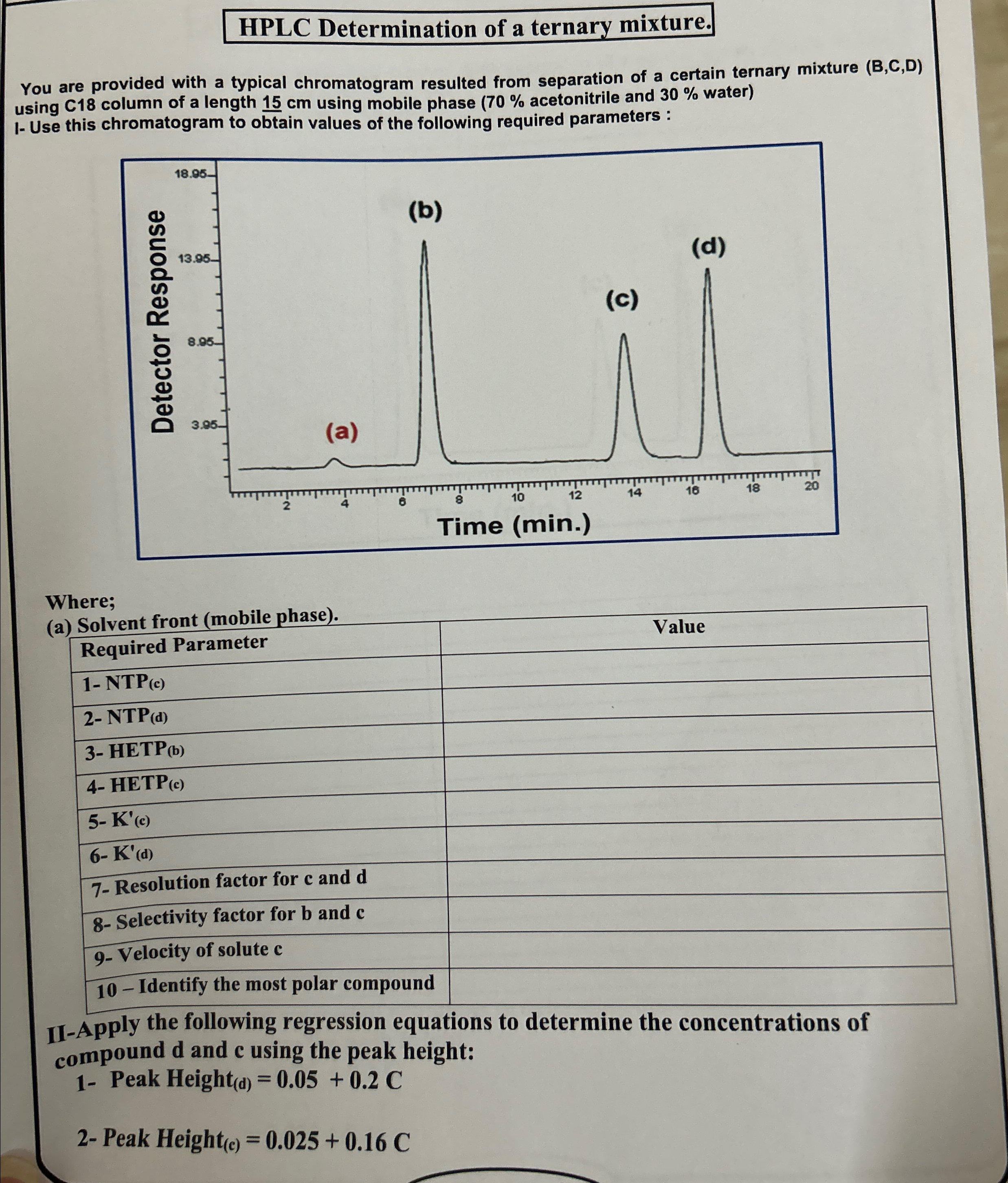
HPLC Determination of a ternary mixture. You are provided with a typical chromatogram resulted from separation of a certain ternary mixture ( B , C
HPLC Determination of a ternary mixture.
You are provided with a typical chromatogram resulted from separation of a certain ternary mixture BCD using column of a length using mobile phase acetonitrile and water I Use this chromatogram to obtain values of the following required parameters :
Where;
a Solvent front mobile phase
tableSequired Parameter, NTPc NTPd HETPb HETPc Kc Kd Resolution factor for c and d Selectivity factor for b and c Velocity of solute c Identify the most polar compound,
IIApply the following regression equations to determine the concentrations of compound and using the peak height:
Peak Height d
Peak Height
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


