Answered step by step
Verified Expert Solution
Question
1 Approved Answer
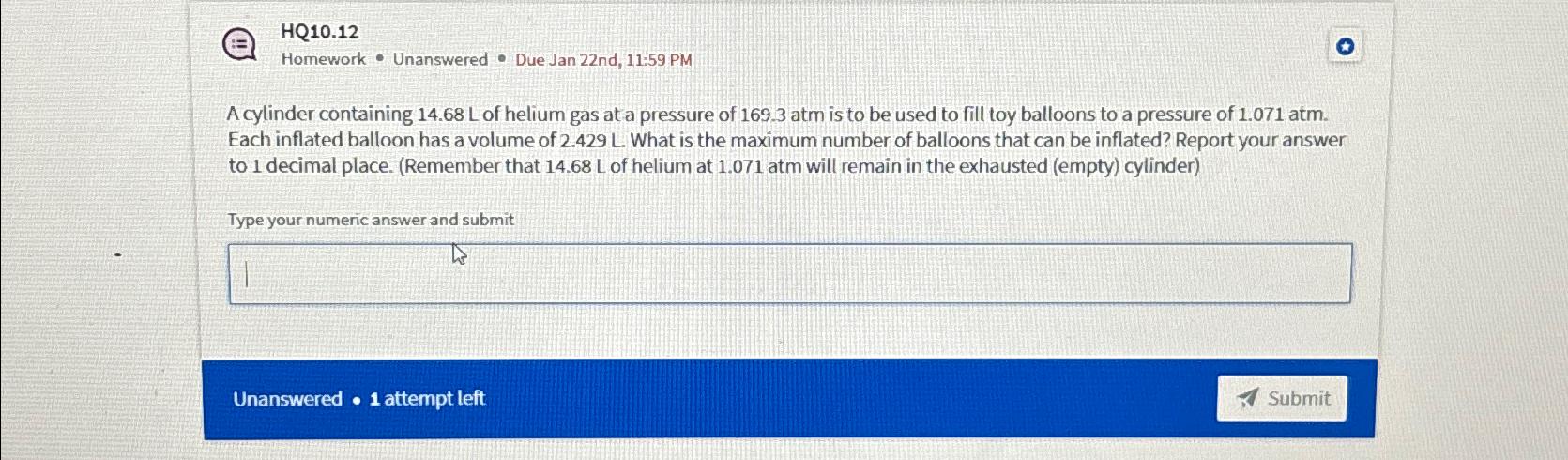
HQ10.12 Homework * Unanswered * Due Jan 22nd, 11:59 PM A cylinder containing 14.68 L of helium gas at a pressure of 169.3atm is to
HQ10.12\ Homework
*Unanswered
*Due Jan 22nd, 11:59 PM\ A cylinder containing 14.68 L of helium gas at a pressure of
169.3atmis to be used to fill toy balloons to a pressure of
1.071atm. Each inflated balloon has a volume of
2.429L. What is the maximum number of balloons that can be inflated? Report your answer to 1 decimal place. (Remember that
14.68Lof helium at
1.071atmwill remain in the exhausted (empty) cylinder)\ Type your numeric answer and submit
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


