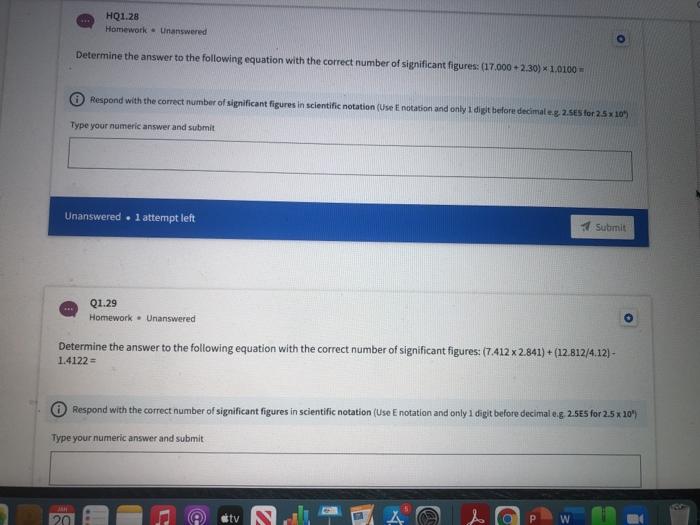
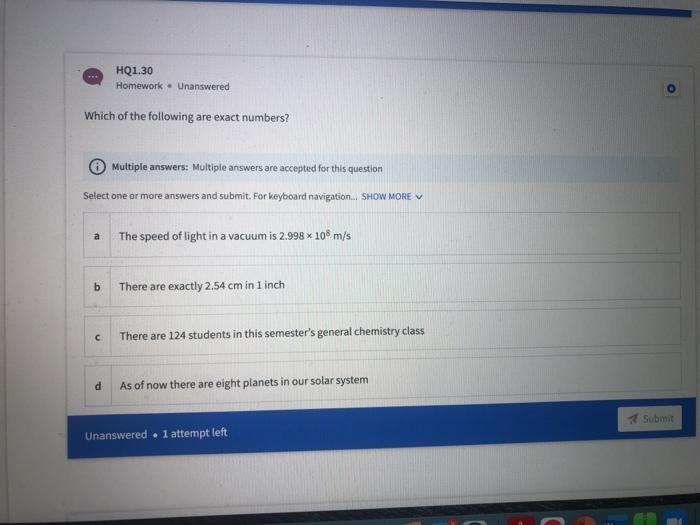
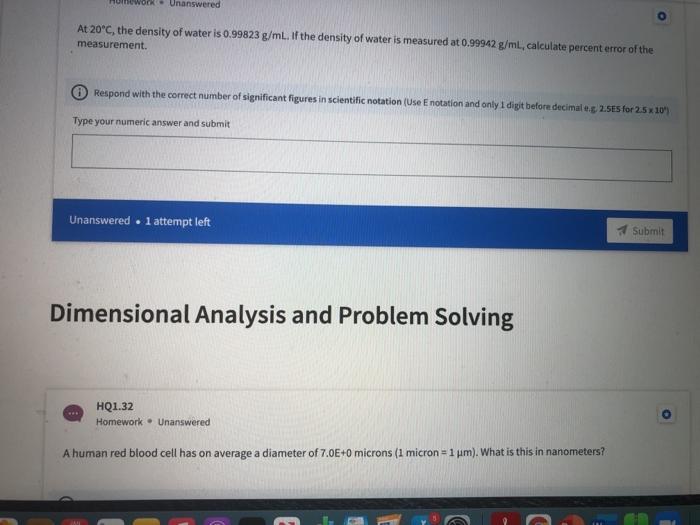
HQ1.36 Homework Unanswered A2.50 gram rectangular object has measurements of 22.0 mm, 13.5 mm, and 12.5 mm. What is the object's density in units of g/cm 37 Do not enter "g/cm3 as part of your answer. (Hint: 1 mm = 0.1 cm) Respond with the correct number of significant figures in scientific notation (Use Enotation and only 1 digit before decimale.g. 2.5E5 for 25 x 10" Type your numeric answer and submit Unanswered . 1 attempt left Submit 48 HQ1.37 Homework Unanswered Calculate the volume (in m3) of a gold object with a mass of 65.8 g given the density of gold is 19.3 g/cm3. Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimale.g 2.5E5 for 2.5 x 10") Type your numeric answer and submit HQ1.38 Homework Unanswered The gas density of water in a room measuring 2.12 m *8.41m *4.89 mis 1.52 ug/cm3. Calculate the mass of water in grams present in the room. Respond with the correct number of significant figures in scientific notation (Use Enotation and only 1 digit before decimale.g. 2.5E5 for 2.5 x 10" Type your numeric answer and submit Unanswered . 1 attempt left Submit HQ1.39 Homework - Unanswered A swimming pool measures 45.0 meters by 30.0 meters. How many grams of water are needed to fill the pool, whose average depth is 2.19 meters? Assume the density of water to be 1.00 g/cm2. Do not enter "grams" as part of your answer. (Hint: 1 cm=0.01 m) Respond with the correct number of significant figures in scientific notation (Use Enotation and only 1 digit before decimaleg 2.5ES for 2.5 x 10" 20 tv A HQ1.34 Homework - Unanswered A substance with a density of 2.70 g/mL occupies a volume of 21.3 ml. What is the mass of the sample in mg? Do not enter "mg" as part of your answer. Respond with the correct number of significant figures in scientific notation (Use Enotation and only 1 digit before decimaleg 2.585 for 2.5x 10" Type your numeric answer and submit Unanswered . 1 attempt left Submit HQ1.35 Homework - Unanswered How many microliters are in 4.00E+1 mL? Do not enter "microliters" as part of your answer. Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimale.g. 2.5E5 for 2.5x10" Type your numeric answer and submit HQ1.32 Homework - Unanswered A human red blood cell has on average a diameter of 7.0E+0 microns (1 micron - 1 um). What is this in nanometers? Respond with the correct number of significant figures in scientific notation (Use Enotation and only 1 digit before decimaleg 2.5E5 for 2.5 x 10" Type your numeric answer and submit Unanswered 1 attempt left Submit HQ1.33 Homework - Unanswered What is the volume in mL of a 1.11 carat diamond, given the density of diamond is 3.51 g/mL? (1 carat = 200 mg). Respond with the correct number of significant figures in scientific notation (Use Enotation and only 1 digit before decimale.g. 2.5E5 for 2.5 10" Type your numeric answer and submit HQ1.28 Homework Unanswered Determine the answer to the following equation with the correct number of significant figures: (17.000+ 2.30) 1.0100 - Respond with the correct number of significant figures in scientific notation (Use Enotation and only I digt before decimaleg 2.5ES for 25x10" Type your numeric answer and submit Unanswered . l attempt left Submit Q1.29 Homework . Unanswered Determine the answer to the following equation with the correct number of significant figures: (7.412 x 2.841) + (12.812/4.12) - 1.4122 Respond with the correct number of significant figures in scientific notation (Use E notation and only i digit before decimal eg 2.585 for 2.5 * 20" Type your numeric answer and submit 20 tv A HQ1.30 Homework Unanswered Which of the following are exact numbers? Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE a The speed of light in a vacuum is 2.998 * 10 m/s b There are exactly 2.54 cm in 1 inch There are 124 students in this semester's general chemistry class d As of now there are eight planets in our solar system Submit Unanswered . 1 attempt left Unanswered At 20C, the density of water is 0.99823 g/ml. If the density of water is measured at 0.99942 g/mL, calculate percent error of the measurement. Respond with the correct number of significant figures in scientific notation (Use Enotation and only I digt before decimaleg 2.5ES for 25 x 10" Type your numeric answer and submit Unanswered . l attempt left Submit Dimensional Analysis and Problem Solving HQ1.32 Homework. Unanswered A human red blood cell has on average a diameter of 7.0E+0 microns (1 micron - 1 um). What is this in nanometers