Answered step by step
Verified Expert Solution
Question
1 Approved Answer
HSP-00-002 ppm The multiplet centered around 1.5ppm belongs to 2 protons and is a partial overlap of two triplets. What structural information does it reveal
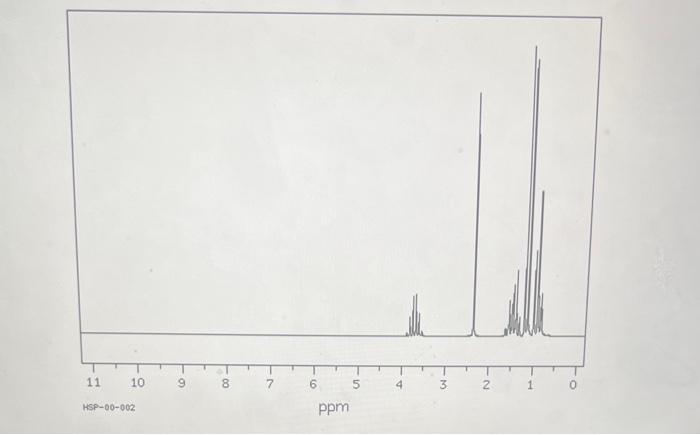


 HSP-00-002 ppm The multiplet centered around 1.5ppm belongs to 2 protons and is a partial overlap of two triplets. What structural information does it reveal about the chemical and shielding environment in which they are located? It belongs to 2H 's on a C bound to two C 's, carrying two H 's, which splits the original signal into a quartet. The protons the quartet belongs to are located in the third most deshielded position on the molecule, which explain why it is in the third most upfield region of the spectrum. It belongs to 2H 's on a C bound to two C's, one carrying two H's, which splits the original signal into a triplet, and the other one carrying one H, which splits each spike of the triplet into a doublet. The neighboring C 's are in different deshielding environment, which explains why the signal is a partially overlapping double triplet. The protons the multiplet belongs to are located in the third least deshielded position on the molecule, which explain why it is in the third most upfield region of the spectrum. It belongs to 2H 's on a C bound to two C 's, one carrying two H 's, which splits the original signal into a triplet, and the other one carrying one H, which splits each spike of the triplet into a quartet. The protons the quartet belongs to are located in the third least deshielded position on the molecule, which explain why it is in the third most downfield region of the spectrum. The peak centered around 2.4ppm belongs to 1 proton. What structural information does it reveal about the chemical and shielding environment in which they are located? It belongs to 1H on an atom bound to an other atom carrying one H, which explains why the signal is a singlet. The proton the singlet belong to are located in the second most deshielded position on the molecule, which explain why it is in the second most downfield region of the spectrum. It belongs to 1H on an atom that precludes interaction of the H with neighboring H 's, which explains why the signal is a singlet. The proton the singlet belongs to is located in the second most deshielded position on the molecule, which explain why it is in the second most downfield region of the spectrum. It belongs to 1H on an atom that precludes interaction of the H with neighboring H 's, which explains why the signal is a singlet. The proton the singlet belongs to is located in the second least deshielded position on the molecule, which explain why it is in the second most downfield region of the spectrum. The multiplet centered around 3.7 ppm belongs to 1 proton. What structural nformation does it reveal about the chemical and shielding environment in which they are located? It belongs to 1H on a C bound on one side to another C carrying two H 's, and on the other side to another C carrying 3H 's, which explains why the signal is a sextet. The proton the sextet belong to is located in the most deshielded position on the molecule, which explain why it is in the most downfield region of the spectrum. It belongs to 1H 's on a C bound to 2 other C's carrying two 3 H's each, which explains why the signal is a sextet. The proton the sextet belong to is located in the most deshiclded position on the molecule, which explain why it is in the most upfield region of the spectrum. It belongs to 1H 's on a C bound on one side to another C carrying two H's, and on the other side to another C carrying 3H 's, which explains why the signal is a quintet. The proton the quintet belong to is located in the most deshielded position on the molecule, which explain why it is in the most upfield region of the spectrum
HSP-00-002 ppm The multiplet centered around 1.5ppm belongs to 2 protons and is a partial overlap of two triplets. What structural information does it reveal about the chemical and shielding environment in which they are located? It belongs to 2H 's on a C bound to two C 's, carrying two H 's, which splits the original signal into a quartet. The protons the quartet belongs to are located in the third most deshielded position on the molecule, which explain why it is in the third most upfield region of the spectrum. It belongs to 2H 's on a C bound to two C's, one carrying two H's, which splits the original signal into a triplet, and the other one carrying one H, which splits each spike of the triplet into a doublet. The neighboring C 's are in different deshielding environment, which explains why the signal is a partially overlapping double triplet. The protons the multiplet belongs to are located in the third least deshielded position on the molecule, which explain why it is in the third most upfield region of the spectrum. It belongs to 2H 's on a C bound to two C 's, one carrying two H 's, which splits the original signal into a triplet, and the other one carrying one H, which splits each spike of the triplet into a quartet. The protons the quartet belongs to are located in the third least deshielded position on the molecule, which explain why it is in the third most downfield region of the spectrum. The peak centered around 2.4ppm belongs to 1 proton. What structural information does it reveal about the chemical and shielding environment in which they are located? It belongs to 1H on an atom bound to an other atom carrying one H, which explains why the signal is a singlet. The proton the singlet belong to are located in the second most deshielded position on the molecule, which explain why it is in the second most downfield region of the spectrum. It belongs to 1H on an atom that precludes interaction of the H with neighboring H 's, which explains why the signal is a singlet. The proton the singlet belongs to is located in the second most deshielded position on the molecule, which explain why it is in the second most downfield region of the spectrum. It belongs to 1H on an atom that precludes interaction of the H with neighboring H 's, which explains why the signal is a singlet. The proton the singlet belongs to is located in the second least deshielded position on the molecule, which explain why it is in the second most downfield region of the spectrum. The multiplet centered around 3.7 ppm belongs to 1 proton. What structural nformation does it reveal about the chemical and shielding environment in which they are located? It belongs to 1H on a C bound on one side to another C carrying two H 's, and on the other side to another C carrying 3H 's, which explains why the signal is a sextet. The proton the sextet belong to is located in the most deshielded position on the molecule, which explain why it is in the most downfield region of the spectrum. It belongs to 1H 's on a C bound to 2 other C's carrying two 3 H's each, which explains why the signal is a sextet. The proton the sextet belong to is located in the most deshiclded position on the molecule, which explain why it is in the most upfield region of the spectrum. It belongs to 1H 's on a C bound on one side to another C carrying two H's, and on the other side to another C carrying 3H 's, which explains why the signal is a quintet. The proton the quintet belong to is located in the most deshielded position on the molecule, which explain why it is in the most upfield region of the spectrum




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


